3001
The Prevalence of Pulmonary Vein Stenosis Post Radio-Frequency Catheter Ablation in Atrial Fibrillation PatientsHana Sheitt1, Julio Garcia1,2, Andrew Howarth1,3, Stephen Wilton1, Carmen P. Lydell1,4, and James A. White1,3
1Stephenson Cardiac Imaging Center, Libin Cardiovascular Institute of Alberta, Calgary, AB, Canada, 2Department of Cardiac Science, University of Calgary, Calgary, AB, Canada, 3Department of Medicine, University of Calgary, Calgary, AB, Canada, 4Diagnostic Imaging, University of Calgary, Calgary, AB, Canada
Synopsis
This study is demonstrating the rule of cardiac MRI in evaluating pulmonary veins (PV) stenosis in atrial fibrillation patients before and after radio-frequency catheter ablation (RFCA).
Synopsis:
This study may be of interest for clinicians and researchers who evaluate pulmonary vein (PV) stenosis in patients with atrial fibrillation (AF) before and after radio-frequency catheter ablation (RFCA). This study demonstrated that: 1) Cardiac MRI before and after RFCA procedure can rule out PV stenosis in patients with paroxysmal AF; 2) Inferior PV are prone to develop stenosis after RFCA.Purpose:
Atrial fibrillation (AF) is a common cardiac arrhythmia associated with significant morbidity and mortality. This is largely related to the increased risk of systemic thromboembolism, including stroke, leading to disability or death. RFCA of the distal PV and posterior left atrium (LA) is increasingly being used by cardiac interventional electrophysiologists to treat patients with AF with a success rate of 60-70% for eradication of the abnormal heart rhythm (1). The success of RFCA is highly dependent on a pre-procedural understanding of the complex three-dimensional (3D) anatomy of the distal PVs and posterior LA as demonstrated by CMR. Neither fluoroscopy nor echocardiography can accurately display the 3D LA anatomy. Cardiac MRI can clearly assess PV ostial dimensions and permit follow-up of the PVs for stenosis. Our study aimed to expand the importance of evaluating PVs before and after RFCA procedure.Methods:
110 subjects (58 with paroxysmal AF referred for pulmonary vein ablation, 42 with RFCA follow-up within 3-6 months, and 10 healthy controls) in an IRB-approved study protocol. Subjects were required to be in sinus rhythm at the time of exam and not have mild mitral insufficiency. Imaging was performed using a 3T MRI scanner (Skyra and Prisma, Siemens, Erlangen, Germany) using a standardized cardiac protocol including a 3D contrast enhanced angiogram (CE-MRA) for the evaluation of PV diameters (A-P: Anterior-Posterior and C-C: Craniocaudal direction) and areas for the left superior (LSPV), left inferior (LIPV), right superior (RSPV), and right inferior (RIPV) PVs. Data analysis was performed using CVI42 (Circle Cardiovascular Imaging Inc., Calgary, Alberta). Left ventriclar function was evaluated using a standard short-axis stack, LA volume was evaluated using bi-planar assessment from 2-chamber and 4-chamber planes (Fig. 1). PV diameters and areas were evaluated using 3D multiplanar reformatting (MPR) (Fig. 2).Results:
All subjects completed the cardiac imaging study and post CE-MRA with good quality. Left ventricular cardiac output (6.5±1.5 L/min vs. 5.8±1.4 L/min, p = 0.014) and right ventricle end-diastolic volume (195±43 ml vs. 174±43 mL, p = 0.029) were decreased after RFCA. All PVs showed a reduced A-P diameter (ANOVA, p<0.05, Fig. 3), as well as RSPV and RIPV C-C (ANOVA, p<0.05, Fig. 3) when comparing groups. Table 2 provide a detailed group comparison for each PV. All PVs also showed decreased areas after RFCA (ANOVA, p≤0.05, Table 3).Discussion and conclusion:
This study demonstrated: 1) the importance of evaluating the PVs before and after RFCA; 2) that inferior PV are prone to develop stenosis after RFCA; 3) a precise pre-procedural understanding of the complex anatomy of the distal pulmonary veins and left atrium will lead to less complications post procedure and will save patients from extra-radiation during the procedure.Acknowledgements
Cardiac Arrhythmia Network of Canada (CANet) AF-START grant, Circle Cardiovascular Imaging Inc., and Mitacs (IT07679 and IT07680).References
1. Cronin P et al. MDCT of left atrium and pulmonary veins in planning radiofrequency ablation for atrial fibrillation: a how-to guide. Am J Roentgenol 2004; 183:767-778.Figures
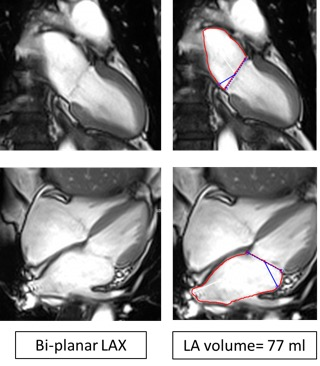
Figure1. Left atrium volumes. Left atrium volume was
obtained using the 2-chamber plane and the 4-chamber plane using a Bi-planar
approach. Volume was calculated by drawing contours around the borders of the
left atrium.
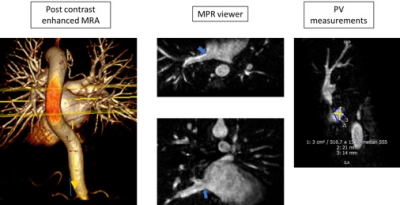
Figure 2. Pulmonary vein measurements. The pulmonary veins
were measured using post contrast enhanced images through a multiplanar
reformatting viewer. Pulmonary veins with the ostial diameters (A-P:
Anterior-Posterior; C-C: Craniocaudal) and circumferences were measured.
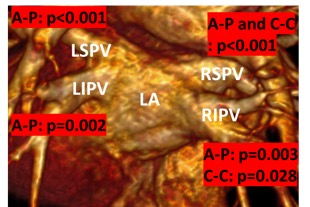
Figure 3. ANOVA group comparison for each pulmonary vein.
LIPV: left inferior pulmonary vein; LSPV: left superior pulmonary vein; RIPV:
right inferior pulmonary vein;
RSPV: Right superior pulmonary vein; A-P:
Anterior-Posterior; C-C: Craniocaudal.
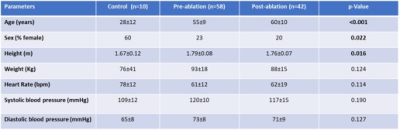
Table 1.
Demographics.
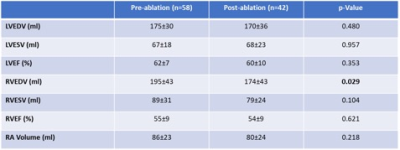
Table 2. Left and ,
right ventricle function and right atrial volumes.
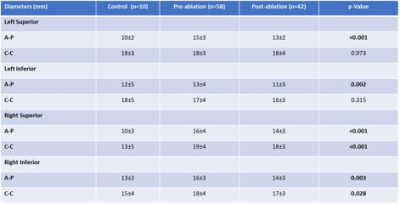
Table 3. Pulmonary
vein diameters by group.
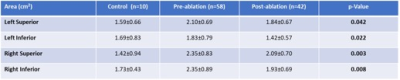
Table 4. Pulmonary
vein areas by group.