2999
Real-time low-field cardiac MRI using an integrated MRI-guided radiotherapy system1Radiation Oncology, Washington University in St Louis, St Louis, MO, United States, 2Computer Science and Engineering, Washington University in St Louis, St Louis, MO, United States, 3ViewRay, Oakwood Village, OH, United States, 4Electrophysiology, Washington University in St Louis, St Louis, MO, United States, 5Biomedical Engineering, Washington University in St Louis, St Louis, MO, United States
Synopsis
The efficacy of stereotactic body radiation therapy (SBRT) cardiac radiosurgery in resolving cardiac arrhythmias was recently reported from a small clinical trial (NCT02919618). However, real-time tracking of the cardiac lesion is challenging using conventional cone-beam CT guided radiotherapy. MRI-guided radiotherapy (MRIgRT) systems integrate real-time MRI for lesion tracking with radiation therapy and can provide excellent cardiac tissue image quality at high frame rates. Real-time cardiac MRI using sparsely-sampled radial acquisitions is demonstrated with iterative reconstruction methods at low-field (0.35 T). The performance goal is to image the heart and track the lesion at 30 Hz with 2.5 mm in-plane resolution.
Purpose
Cardiac arrhythmias are a major cause of hospitalizations, morbidity, and mortality.1 Ventricular tachycardia (VT) patients who have failed standard treatments have a one-year survival of 20%.
An ongoing Phase I/II clinical trial (NCT02919618) at our institute is using stereotactic image-guided radiotherapy (IGRT) to treat refractory ventricular tachycardia (VT), the principle cause of sudden cardiac arrest. Preliminary results have shown a high success rate for the treatment with minimal side effects. The stereotactic radiosurgery is currently being performed using cone-beam CT IGRT (CBCT-IGRT) without real-time tracking.
In 2017, FDA-approved commercial MRI-Linacs became available. MRI-guided radiotherapy (MR-IGRT) offers superior soft tissue contrast to CBCT-IGRT, fast imaging without ionizing radiation, and outstanding cardiac image quality at low magnetic field strengths. Knowledge of heart motion should enable radiation oncologists to more accurately target lesions on the heart for cardiac radiosurgery.
The objective of this study was to acquire real-time images of the heart on a MR-IGRT system at a sampling rate of 30 Hz with an in-plane spatial resolution of <2.5 mm to accurately characterize cardiac and target lesion motion.2 The information can be used either for cardiac dose sparing (e.g., for MR-IGRT of the lung or breast) or targeting (e.g., for stereotactic cardiac radiosurgery).
Methods
Healthy volunteers received cardiac MRI on a 0.35 T ViewRay MRIdian (Oakwood Village, OH) 60Co MR-IGRT system after providing informed consent. The MRIdian pulse sequences were compiled using Siemens IDEA/ICE (VB19). Volunteers were positioned supine on top of a torso phased array receive coil. A second torso coil was placed on top of the volunteer. Each coil contained 6 receive channels.
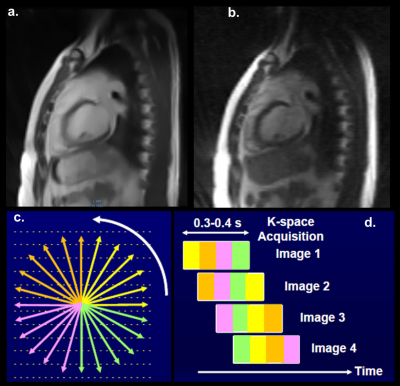
2D sagittal radial TrueFISP MRIs were acquired of the heart with the MRIdian operating in MRI-only mode. The sequences acquired 10-100 radials with acquisition times ranging from 28 ms/image to 280 ms/image. By comparison, real-time therapy tracking on the MR-IGRT is performed at 0.25 s/image with an in-plane resolution of 3.5 mm while 3D treatment planning MRI scans are acquired in 17-360 s at an in-plane resolution of 1.5-1.6 mm. ViewRay is also testing a radial 2D sequence with a compressed sensing reconstruction that uses k-space sharing with a temporal resolution of <100 ms/image.3
Our radial acquisitions were reconstructed using Siemens default filtered backprojection (FBP) reconstruction, our own FBP algorithm, and iterative reconstructions including fast iterative thresholding algorithm with a regularizer using the L1 norm of the wavelet coefficients of the image (FISTA-wL1)4,5, the optimized parallel imaging L1-ESPIRiT6 algorithm using the Berkeley advanced reconstruction toolbox, and the latter further optimized using wavelet regularization, all in an effort to reduce streaking artifacts resulting from k-space undersampling.
Results
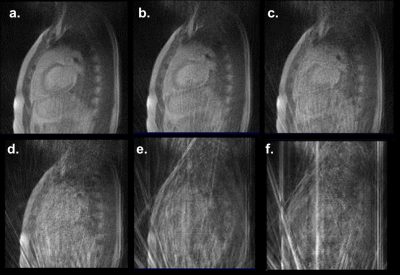
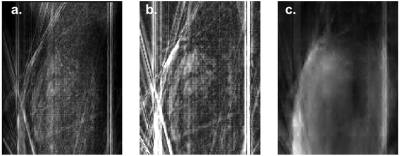
The image quality for the radial acquisitions using Siemens FBP image reconstructions are compared in Fig. 1. The image quality degrades rapidly for acquisition times below 100 ms/image and particularly for the target of 30 ms/image (e.g., 10 radials).
ViewRay's radial sequence (112 radials) with k-space sharing and proprietary compressed sensing reconstruction produces high quality images in real-time on the MRI system (Fig. 2a). However, our 100 radial acquisition images reconstructed using FISTA-wL1 (Fig. 2b) appear less smoothed than ViewRay's and are vastly improved from the FBP reconstruction (Fig. 1a).
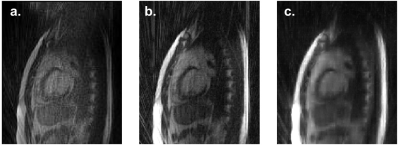
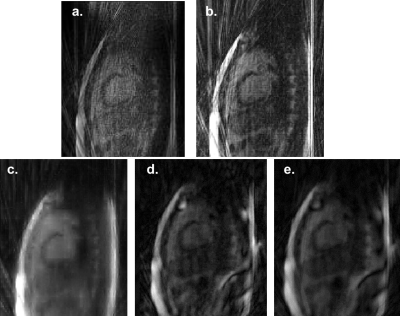
The FISTA-wL1 reconstruction also produced superior images compared to Siemens' or our FBP reconstructions (Figs. 3-5). The heart features are clear with an acquisition of 20 radials but are unsatisfactory for 10 radials (Figs. 4-5).
Discussion
The FISTA-wL1 reconstruction algorithm is promising. The images reconstructed using FISTA-wL1 were superior to our ESPIRiT and compressed sensing algorithms (Fig. 5). However, FISTA-wL1 was not able to reconstruct images from acquisitions with 10 radials. Ultimately, we want to adapt our reconstruction algorithms to handle real-time 3D acquisitions with severe undersampling.
Our image reconstructions were performed offline. The algorithms would have to be implemented in Siemens image calculation environment (ICE) or the k-space data would have to be ported to a powerful processor in real-time real-time tracking. However, ViewRay currently performs their own real-time image processing so we are not concerned with the practicality of the eventual implementation for real-time motion tracking.
Conclusion
High quality cardiac cine MRIs were successfully acquired at low-field using radial TrueFISP without k-space sharing. However, further enhancements in image acquisition and reconstruction are required in order to meet the 30 Hz sampling rate target with adequate image quality and spatial resolution.Acknowledgements
This research was supported by Washington University in St. Louis’s Department of Radiation Oncology.References
1. Vroomen M, Pison L. Hybrid ablation for atrial fibrillation: a systematic review. J Interv Card Electrophysiol 2016;47(3):265-274.
2. Bernstein MA, King KF, Zhou XJ. Handbook of MRI Pulse Sequences. Amsterdam: Elsevier; 2004.
3. Gach HM, Nana R, Robinson CG, Cuculich PS, Kashani R, Bradley JD, Roach MC, Dempsey JF, Mutic S, Green OL. Low-field cardiac MRI for cardiac radiosurgery using an integrated MRI-guided radiotherapy system. 2017; Honolulu, HI.
4. Beck A, Teboulle M. A fast iterative shrinkage-thresholding algorithm for linear inverse problems. SIAM J Imaging Sciences 2009;2(1):183-202.
5. J. A. Fessler, “Michigan Image Reconstruction Toolbox”, available at https://web.eecs.umich.edu/~fessler/code/index.html.
6. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med 2014;71(3):990-1001.
Figures