2996
Free Breathing Multiple Delays Renal Perfusion MRI using Hadamard encoded pCASL1Global MR Applications & Workflow, GE Healthcare, Tokyo, Japan, 2Radiological Center, University of Fukui Hospital, Fukui, Japan, 3Department of Radiology, University of Fukui, Fukui, Japan, 4Global MR Application & Workflow GE Healthcare, Calgary, AB, Canada
Synopsis
Current pCASL renal perfusion imaging is typically restricted to a single post label delay (PLD) time. While multiple PLD (mPLD) times can be achieved with sequential scans with different PLD times, this procedure is time consuming. A rapid acquisition was developed using Hadamard encoding for mPLD pCASL imaging combined with a motion robust timing and readout strategy to permit free breathing renal ASL. The feasibility study explores the application of Hadamard encoding to renal perfusion imaging where spin labeling is affected by pulsatile flow and demonstrated that a cardiac triggered scan provided stable perfusion images achieving ATT corrected renal blood flow with seven PLD acquisition
Purpose
Renal perfusion MRI with Arterial Spin Labeling (ASL) has shown promise on evaluating renal functionality1. One of the current main technical challenges is respiratory motion that causes image subtraction error between label and control image. A variety of strategies for respiratory motion compensation have been proposed, including multiple breath-holding, bellows respiratory and navigator echo based motion correction2,3,4. These mitigation strategies tend to be inefficient in terms of scan time because of selecting only the acquired data in good condition, which resulting in being difficult to allow multiple post labeling delay imaging that requires many ASL dataset acquisitions. In addition, to increase reproducibility of perfusion quantification, arterial transit time (ATT) that changes between individuals and regions should be preferably taken into consideration in calculation of renal blood flow (RBF). In this work, accelerated acquisition of multiple point delay perfusion maps was performed using free-breathing Hadamard encoded scan. The effect of signal averaging and pulsatile flow was investigated.Methods
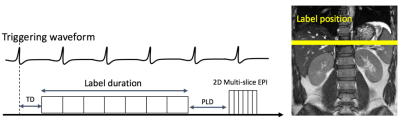
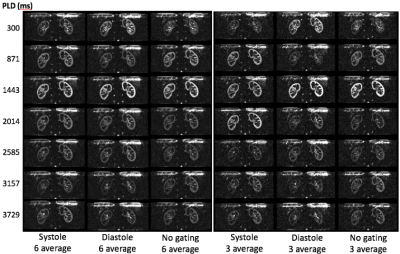
ASL was performed using pseudo-Continuous ASL (pCASL) where Hadamard time encoded labeling was applied by splitting label duration into sub-boli (Fig.1). Data was acquired using multi-slice 2D single shot SE-EPI with half Fourier and parallel imaging (ssEPI+PI). Background suppression was implemented using 3 inversion pulses at 3000ms, 1000ms and 200ms from image acquisition. ASL datasets were repeatedly acquired to increase motion robustness by averaging effect and to boost SNR of renal signal. After imaging reconstruction, each slice images were aligned with the reference image that was averaged image from all the acquired slice dataset. Then the realigned images were decoded by applying Hadamard matrix into sub-bolus images, which were averaged to form mean perfusion weighted image (PWI). A volunteer was scanned on a 3.0T Scanner (Discovery MR 750, GE Healthcare, Waukesha, WI, U.S.A.) with 32 channel body receiver array coils under IRB approval. The following scan parameters were used: Coronal scan plane, 2D SE-EPI sequence, TR/TE = 7000-9000/17.8 msec, Matrix = 96x128, #slices = 4, FOV = 38x38 cm, slice thickness = 8.0mm. Labeling position was prescribed graphically with 3 mm thickness on upper region of kidney in axial plane to be insensitive to B0 inhomogeneity. Seven blocks of 571 ms labeling duration (LD) and postlabeling delays (PLD) of 300, 871, 1443, 2014, 2585, 3157, and 3729 ms were used. To investigate the effect of pulsatile flow, ASL data sets were acquired for 1) non-triggered pCASL 2) systolic triggered pCASL 3) diastolic triggered pCASL synchronized with peripheral pulse signal. To assess the signal averaging effect, 3 averaging and 6 averaging were compared for multiple PLD and RBF map. 3 average dataset was generated from 6 average dataset. For renal quantification, ATT and ATT-corrected RBF were calculated5 comparing the signal weighted delay of ΔM with theoretical signal curves.Results
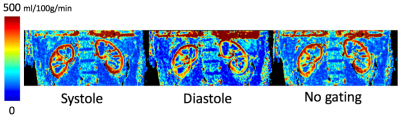
All the multiple delay renal perfusion images were successfully acquired for systole (8:18), diastole (8:49) and no gating (6:18). Figure 2 shows seven delayed perfusion weighted images to compare the effect of pulsatile flow in three types of cardiac triggering showing similar signal peak timing at PLD=1443 ms and different bolus profile in time course of multiple PLDs. The number of signal averaging affected the bolus profile with increased variation appearing cortex signal even at PLD=3729 in the systolic triggering and the no gating. ATT corrected RBF map was calculated (Fig.3) showing the highest value of RBF in systolic trigger. RBF map between 3 averages and 6 averages were almost the same. Signal outliers of PWI and RBF map at the edge of the kidney were not seen because of voxel mismatch among tag, control images due to respiratory motion.Discussions
This feasibility study has shown that the diastolic triggering scan gives stable multi PLD maps while systolic triggering and no gating scan include image artifact on the PLD images widening bolus profile in time course with increase of number of averaging. Cardiac triggered pCASL allows regular cardiac cycle in label duration where Hadamard encoding reconstruction can avoid artifact on the resultant sub-boli ASL images. By selecting appropriate cardiac triggering, accurate RBF map should be calculated from artifact free multi PLD maps. In addition, smaller number of averaging is available to reduce scan time as it is shown that 3 averages and 6 averages give almost the same RBF map.
Conclusion
We have developed multiple PLD renal perfusion imaging using Hadamard encoded pCASL with motion robust, rapid acquisition and patient comfort to obtain dynamic PWI information and RBF with ATT correction. Appropriate cardiac triggering is taken into consideration to reduce the sensitivity of pulsatile flow.
Acknowledgements
No acknowledgement found.References
1. Taylor AT. Radionuclides in nephrourology, part 2: pitfalls and diagnostic applications. J Nucl Med 2014; 55: 786–798
2. Robson PM, Madhuranthakam AJ, Dai W, Pedrosa I, Rofsky NM, Alsop DC. Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling. Magn Reson Med 2009; 61:1374–1387.
3. Gardener AG, Francis ST. Multislice perfusion of the kidneys using parallel imaging: image acquisition and analysis strategies. Magn Reson Med 2010;63:1627–1636.
4. Tan H, Koktzoglou I, Prasad PV. Renal perfusion imaging with two- dimensional navigator gated arterial spin labeling. Magn Reson Med 2014;71:570–579.
5. Dai W, Robson PM, Shankaranarayanan A, Alsop DC. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med 2012; 67:1252–1265.
Figures