2990
Retrospective Multi-Phase Non-Contrast-Enhanced Magnetic Resonance Angiography (ROMANCE MRA) for Robust Angiogram Separation in the Presence of Cardiac Arrhythmia1Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Republic of Korea, 2Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Helen Wills Neuroscience Institute, University of California, Berkeley, CA, United States, 4Department of Radiology, Gachon University Gil Medical Center, Incheon, Republic of Korea, 5Department of Radiology, Seoul National University Hospital, Seoul, Republic of Korea
Synopsis
In the proposed ROMANCE MRA, data were continuously acquired
Intorduction
Non-contrast-enhanced (NCE) magnetic resonance angiography (MRA) using cardiac-gated fresh blood imaging (FBI)1-3 exploits a pulsatile nature of arterial blood flow to differentiate arteries from veins and stationary background tissues. Two sets of volumetric images in cardiac systolic and diastolic phases are subtracted to yield angiograms while suppressing veins and background tissues. To maximize arterial signal intensities after subtraction, arterial signal intensities with varying cardiac phases are to be estimated prior to imaging to determine optimal trigger delay times for subject-specific, systolic and diastolic phases. Nevertheless, if discrepancies in cardiac activities between the pre-scout and the imaging scans occur, this problem still remains unsolved. Particularly in patients with cardiac arrhythmia, conventional FBI methods would fail to produce proper angiograms because it is very difficult to acquire the two sets of data in desired cardiac phases, respectively. Irregular heartbeats in subjects may lead to varying effective times of repetition (TR) between the two separate acquisitions in cardiac systole and diastole, potentially yielding substantial difference in background signal intensities between the two scans. Thus, conventional FBI suffers from incomplete background suppression after subtraction. Additionally, whenever R-R intervals are not long enough to accommodate both trigger delay time and data acquisition window, an effective TR is extended over multiple heartbeats, prolonging total imaging time. As an approach to eliminate a separate acquisition of the systolic and diastolic data as well as a pre-scout scan for optimal selection of trigger delay times we develop a novel, RetrOspective Multi-phAse Non-Contrast-Enhanced (ROMANCE) MRA in a single acquisition for robust angiogram separation even in the presence of cardiac arrhythmia.
Materials and Methods
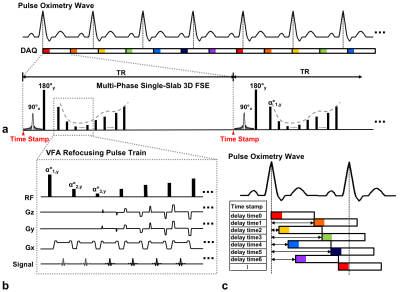
Spatially-selective single-slab variable-flip-angles 3D FSE in sync with an external pulse oximeter is employed due to its inherent sensitivity to blood flow and high encoding efficiency4,5 (Fig1a,b). To facilitate multi-phase data acquisition, a constant TR, which is asynchronous with a subject-specific cardiac cycle, is chosen to avoid an integer multiple of an average R-R period. Real-time information on cardiac cycles from the pulse oximetry including the starting time points of each excitation RF pulse from the last occurrence of R-wave is continuously recorded over the entire scan.6 Given an average R-R period, a time series set of volumetric data is retrospectively rearranged and positioned into equidistant, temporal bins (k-bin space) to produce flow-sensitive, time-varying arterial signals such that angiograms are distributed over all cardiac phases (Fig1c).
Multi-phase, flow-sensitive images ($$$\mathbf{X} $$$) in x-bin space can be decomposed into: 1) a baseline ($$$\mathbf{\overline{X}}$$$) that contains similar anatomical structures, and 2) its corresponding residual ($$$\boldsymbol{\Delta}\mathbf{X}$$$) that reflects time-varying angiograms. $$\mathbf{X=\overline{X}+\boldsymbol{\Delta}X}$$ We estimate time-varying angiograms directly from highly undersampled k-bin space by solving the following constrained optimization problem with both subtraction-induced sparsity and low rank priors.
$$\mathrm{\boldsymbol{\Delta}\widehat{\mathbf{X}}=arg\;\underset{\mathbf{{\Delta}X}}{min}\left\|\boldsymbol{\mathcal{P}_s}\left(\boldsymbol{\Delta}\mathbf{X}\right)\right\|_{1}+\lambda_{\ell}\left\|\boldsymbol{\mathcal{P}_{\ell}}\left(\boldsymbol{\Delta}\mathbf{X}\right)\right\|_*}\\{s.t.\left\|\mathrm{\mathbf{v-E}\boldsymbol{\left(\boldsymbol{\Delta}\mathbf{X}\right)}}\right\|_2<\sigma^2}$$
where $$$\mathbf{v}=\mathbf{y}-\mathbf{E}\left(\mathbf{\overline{X}}\right)$$$, $$$\mathbf{y}$$$ is measured data, $$$\mathbf{E}\left(\cdot\right)$$$ is sensitivity encoding operator, $$$\boldsymbol{\mathcal{P}_s}\left(\cdot\right)$$$ is sparsifying transfrom applied to the bin dierction, $$$\boldsymbol{\mathcal{P}_{\ell}}\left(\cdot\right)$$$ is two-step operator that applies the spatial Fourier transform followed by the Hankel operator, $$$\lambda_{s}$$$ and $$$\lambda_{\ell}$$$ are penalty parameters that balances between sparsity and low rank terms, and $$$\sigma^2$$$ is the noise variance that controls the fidelity of the estimates to the measured data.
Experimental studies were performed on a 3T Trio system (Siemens, Erlangen, Germany). Peripheral MRA was performed in a normal volunteer and a volunteer with cardiac arrhythmia using conventional FBI and the proposed ROMANCE MRA for comparison. Imaging parameters are shown in Table1.
Results
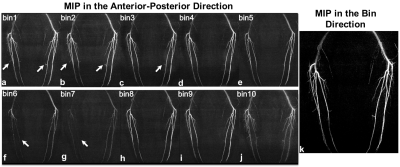
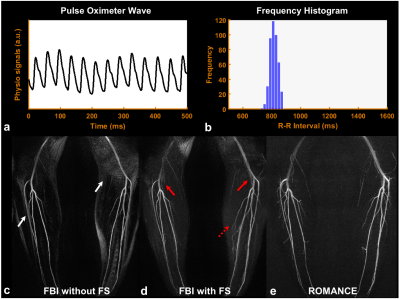
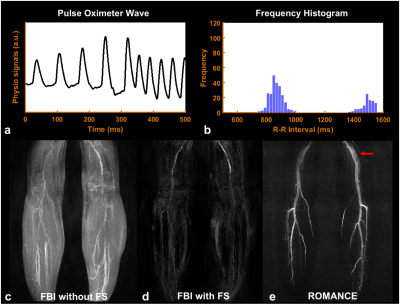
Figure 2 shows angiograms in each bin (Figs.2a-2j) and the corresponding, final angiogram projected along bin direction (Fig.2k) using the proposed ROMANCE MRA. Figures 3 and 4 show physiological signals from the pulse oximeter, the corresponding histogram of R-R intervals, and the resulting angiogram estimates from normal healthy volunteer and a volunteer with highly irregular heartbeats. Conventional FBI without/with fat saturation (FS) delineates major peripheral arteries fairly well (Figs.3c,3d), and the proposed ROMANCE MRA depicts even small branching arteries with further suppression of background artifacts (Fig.3e). Despite cardiac arrhythmia, the proposed method outperforms conventional methods in delineating peripheral angiograms with robust background suppression (Fig.4e).
Discussion and Conclusion
In the presence of irregular heartbeats (cardiac arrhythmia), the proposed ROMANCE MRA acquisition is of great value in addressing the problems related to arterial signal loss and background artifacts in conventional methods. Furthermore, the proposed method can be interpreted as a completely generalized version of conventional FBI in that the former utilizes images over the entire cardiac phase to produce angiograms while the latter employs images only in the two cardiac phases. The proposed method is expected to widen the utilities of the NCE MRA in a clinical routine.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science (2016M3C7A1913844, NRF-2017R1A2B4012581)References
1. Miyazaki M, Sugiura S, Tateishi F, Wada H, Kassai Y, Abe H. Non-contrast-enhanced MR angiography using 3D ECG-synchronized half-Fourier fast spin echo. J Magn Reson Imaging 2000; 12: 776-783.
2. Miyazaki M, Takai H, Sugiura S, Wada H, Kuwahara R, Urata J. Peripheral MR angiography: Separation of arteries from veins with flow-spoiled gradient pulses in electrocardiography-triggered three-dimensional half-Fourier fast spin-echo imaging. Radiology 2003; 227: 890-896.
3. Fan Z, Sheehan J, Bi X, Liu X, Carr J, Li D. 3D noncontrast MR angiography of the distal lower extremities using flow-sensitive dephasing (FSD)-prepared balanced SSFP. Magn Reson Med 2009; 62: 1523-1532.
4. Busse RF. Flow sensitivity of CPMG sequences with variable flip refocusing and implications for CSF signal uniformity in 3D-FSE imaging. In Proceedings of the 14th Annual Meeting of ISMRM, Seattle, WA, USA. 2003; p 2430.
5. Mugler JP. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging 2014; 39: 745-767.
6. Mendes J, Parker DL, Hulet J, Treiman GS, Kim SE. CINE turbo spin echo imaging. Magn Reson Med 2011; 66: 1286-1292.
Figures