2989
Artifact Reduction in 3D Radial Whole-Heart Imaging Using Slab-Selective RF Excitation1Siemens Medical Solutions USA Inc, Chicago, IL, United States, 2Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 3Siemens Healthcare, Erlangen, Germany
Synopsis
To date, most 3D radial kooshball imaging implementations had used non-selective (NS) radiofrequency pulses for volumetric excitation. However, given the undersampled nature of radial imaging, signal from excited regions in the periphery increases the streaking level in the central area of the field-of-view. In this work, we implemented slab-selective (SS) excitation for 3D radial whole-heart imaging. Results on 10 volunteers showed that SS excitation improved mean apparent signal- and contrast-to-noise ratio by 24% and 40%, respectively, with a mean scan time increase of 26% due to longer TR.
Background
3D radial kooshball imaging has been shown to be a suitable method for whole-heart imaging due to its wide coverage, inherent isotropic resolution, and robustness to motion. Previous investigations have tested this methodology in several applications including coronary magnetic resonance angiography1-3, late gadolinium enhancement imaging4, and myocardial parametric mapping5. To date, most implementations make use of non-selective (NS), hard radiofrequency (RF) pulses for efficient volumetric excitation. However, a typical 3D radial k-space trajectory is highly undersampled from what is required by the Nyquist criterion. As a result, the diameter of the alias-free region in the point-spread-function (PSF) is much smaller than the NS excited volume, which can reach or exceed the size of the imaging field-of-view (FOV) . As a result, signal from the periphery, such as the shoulders, the neck, and the abdomen, is convolved with the PSF and increases the streaking artifact level in the central FOV. In this work, we hypothesize that slab-selective (SS) excitation will reduce such artifacts in 3D radial whole-heart imaging.
Methods
A prototype ECG-gated, fat-suppressed, and T2-prepared 3D radial whole-heart self-navigated sequence with balanced steady-state free precession readout2 was adapted to enable SS excitation through decoupling a fixed excitation and a rotating readout orientations. The imaging parameters were as follows: TE/TR = 1.5/3.0 ms and 1.9/3.8 ms for NS and SS excitation, respectively, flip angle = 115°, transversal SS excitation thickness = 165 mm, T2 preparation duration = 40 ms, FOV = (220 mm)3, matrix size = (192 mm)3, spatial resolution = (1.15 mm)3, total number of radial views = 11,908-12,224 , views per heartbeat = 25-32 to match the acquisition window durations of the NS and SS scans to preserve fat-saturation effectiveness and minimize cardiac motion. Phantom study and whole-heart in vivo studies on healthy volunteers (N=10) with IRB approval and written informed consent were performed on 1.5T clinical scanners (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). The apparent signal-to-noise ratio (aSNR) of the blood pool and contrast-to-noise ratio (aCNR) between blood and myocardium were calculated from the blood pool signal, measured from a circular region-of-interest (ROI) in the aorta at the level of the left coronary ostium, the myocardium signal, and the apparent noise level defined as the signal standard deviation (SD) in an ROI placed in the nearby lung, as shown in fig. 1. Statistical tests were performed using Student’s paired t-test.
Results
Suppression of the periphery signal and the reduced artifact level from SS excitation is demonstrated in the phantom example reconstructed inline shown in Fig. 2, and the retrospectively reconstructed in vivo example in Fig. 3, where the entire oversampled FOV is shown without cropping. All volunteer experiments were completed successfully. The mean imaging time with NS and SS excitation were 6.3±0.7 and 7.9±0.9 minutes (P<0.001), respectively; the acquisitions took on average 380±2 and 475±14 heartbeats (P<0.001), respectively; the mean aSNR values were 20.9±4.3 and 26.0±6.1 (P=0.013), respectively; the mean aCNR values were 10.3±3.6 and 14.4±4.2 (P=0.006), respectively. Consistent with the quantitative measurements, streaking artifacts were significantly reduced in all volunteers, exemplarily shown in two subjects in Figs. 4 and 5.
Discussion
In this work, we implemented SS excitation for 3D radial whole-heart imaging and performed preliminary comparison with a more conventional NS excitation. Our results showed that SS excitation, which reduces streaking artifact contribution from the periphery signal, improves mean aSNR and aCNR significantly by 24% and 40%, respectively. Due to the longer RF pulse, TR of the SS protocol was approximately 26% longer than the NS protocol. The number of views acquired per acquisition window in the SS protocol was reduced to maintain the fat-saturation and cardiac motion suppression efficacy as in the NS protocol. To mitigate the extra scan time required, shorter RF pulse options may be explored, especially because the slab profile is not critical in 3D radial imaging due to the built-in oversampling in all three directions. One may also take advantage of the lower streaking level of the SS protocol and experiment additional undersampling and iterative reconstruction schemes to further reduce the total acquisition time. Other imaging orientations, such as sagittal or coronal, may also be explored for optimal periphery signal suppression.
Conclusion
The results in this study show that SS excitation improved the image quality of 3D radial whole-heart imaging over the conventional NS excitation. Future work is desired to address the tradeoff in imaging time.
Acknowledgements
No acknowledgement found.References
Stehning, C., Börnert, P., Nehrke, K., Eggers, H. and Stuber, M. (2005), Free-breathing whole-heart coronary MRA with 3D radial SSFP and self-navigated image reconstruction. Magn. Reson. Med., 54: 476–480. doi:10.1002/mrm.20557
Piccini, D., Littmann, A., Nielles-Vallespin, S. and Zenge, M. O. (2012), Respiratory self-navigation for whole-heart bright-blood coronary MRI: Methods for robust isolation and automatic segmentation of the blood pool. Magnetic Resonance Medicine, 68: 571–579. doi:10.1002/mrm.23247
Pang, J., Bhat, H., Sharif, B., Fan, Z., Thomson, L. E. J., LaBounty, T., Friedman, J. D., Min, J., Berman, D. S. and Li, D. (2014), Whole-heart coronary MRA with 100% respiratory gating efficiency: Self-navigated three-dimensional retrospective image-based motion correction (TRIM). Magn. Reson. Med., 71: 67–74. doi:10.1002/mrm.24628
Rutz, T., Piccini, D., Coppo, S., Chaptinel, J., Ginami, G., Vincenti, G., Stuber, M. and Schwitter, J., 2016. Improved border sharpness of post-infarct scar by a novel self-navigated free-breathing high-resolution 3D whole-heart inversion recovery magnetic resonance approach. The international journal of cardiovascular imaging, 32(12), pp.1735-1744.
van Heeswijk, R. B., Piccini, D., Feliciano, H., Hullin, R., Schwitter, J. and Stuber, M. (2015), Self-navigated isotropic three-dimensional cardiac T2 mapping. Magn. Reson. Med., 73: 1549–1554. doi:10.1002/mrm.25258
Figures
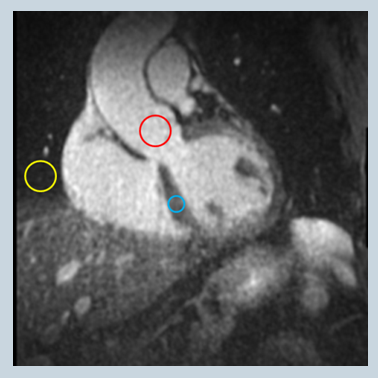
Figure 1: signal and apparent noise ROI placement; red: blood; blue: myocardium; yellow: apparent noise
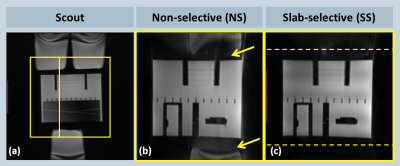
Figure 2: Phantom experiment comparing NS and SS excitation when the object is larger than the prescribed FOV; (a) coronal scout image; box and line indicate the prescribed 3D radial imaging volume and the location of the sagittal slice shown on the right; (b) NS excitation shows considerable artifacts (arrows) from peripheral, out-of-FOV signal sources; (c) SS excitation effectively suppresses such signal and reduces aliasing in the central FOV; dashed lines indicate edges of the prescribed excitation slab.
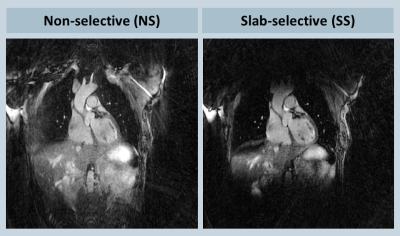
Figure 3: In-vivo experiment comparing NS and SS excitation; (a) NS excitation shows elevated artifact level from the periphery; (b) SS excitation effectively suppresses peripheral signal and reduces aliasing in the central FOV.
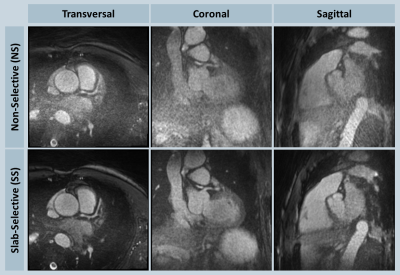
Figure 4: In vivo NS-SS comparison in one volunteer shown in transversal, coronal and sagittal orientations; in this example, strong streaking artifacts from the abdominal region were seen with the NS excitation, which significantly elevated the background aliasing of the heart region; slab-selective excitation improved the image quality by effectively suppressing such signal sources.
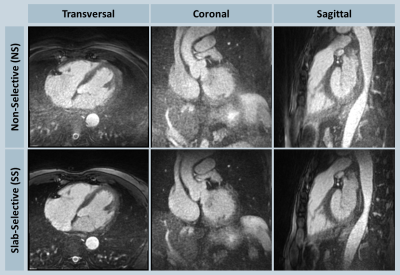
Figure 5: In vivo NS-SS comparison in another volunteer shown in transversal, coronal and sagittal orientations; overall higher background aliasing was observed with NS excitation, whereas SS excitation improved the image quality considerably.