2977
High-resolution Imaging with a priori Knowledge Incorporating the Navier-Stokes equations and the discontinuous Galerkin method (4D flow HIKING): towards flow reconstruction constrained by computational fluid dynamics1Diagnostic Radiology, Department of Clinical Sciences, Skane University Hospital, Lund University, Lund, Sweden, 2Department of Mathematics, Lawrence Berkeley Laboratory and University of California, Berkeley, CA, United States, 3Lund University Bioimaging Center, Lund University, Lund, Sweden, 4Clinical Physiology, Department of Clinical Sciences, Skane University Hospital, Lund University, Lund, Sweden
Synopsis
Magnetic resonance 4D flow imaging is a promising technique for diagnosis and follow-up of disease. However, 4D flow is limited by long scan times and low resolution. This work presents phantom validation of a new method for 4D flow scan acceleration, called 4D flow high-resolution imaging with a priori knowledge incorporating the Navier-Stokes equations and the discontinuous Galerkin method (4D flow HIKING). Excellent agreement with laser particle image velocimetry (PIV) was found, demonstrating the potential of the framework for scan time reduction and enhanced data quality in 4D flow.
Background
Recent accelerated MRI methods exploit a priori information, e.g. spatiotemporal correlations (1) or sparsity (2). The Navier-Stokes equations describe blood flow physics accurately and can therefore give valuable a priori information for 4D flow, promising increased data quality and/or reduced scan times. However, previous Navier-Stokes 4D flow reconstruction methods are limited by simplified treatment of the Navier-Stokes equations (3) and insufficient modelling of spatiotemporal smoothing in MRI (4).
We have recently developed a 4D flow reconstruction framework, called 4D flow high-resolution imaging with a priori knowledge incorporating the Navier-Stokes equations and the discontinuous Galerkin method (4D flow HIKING), including a high-order computational fluid dynamics (CFD) solver and a spatiotemporal smoothing model. Therefore, this study aims to 1) validate 4D flow HIKING in a phantom setup with laser particle image velocimetry (PIV) as the reference standard and 2) investigate the performance of 4D flow HIKING in the presence of noise and low resolution in synthetic 2D data.
Methods
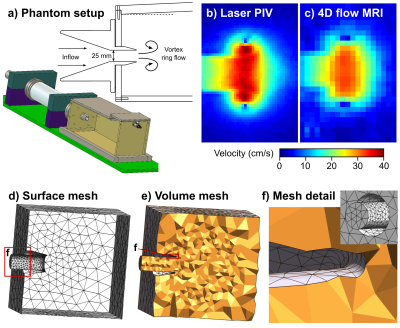
For the phantom validation, a previously presented setup based on vortex ring formation was used (5) (Figure 1). A pulsatile pump drives flow through a 25 mm nozzle to generate vortex rings, a highly repeatable flow condition modelling diastolic flow in the human heart (6).
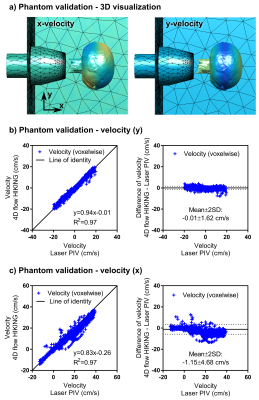
A computational mesh with approximately 50,000 tetrahedral elements (p=2) was used for the 4D flow HIKING reconstruction (Figure 1d-f, Figure 2a). The input data to the 4D flow HIKING reconstruction consisted of the computational mesh and low-resolution 4D flow data obtained using conventional SENSE reconstruction (Philips Achieva 1.5T, Best, The Netherlands) (5). The inflow in the tank nozzle was assumed to be constant over the nozzle, and parameterized by a Gaussian profile in time yielding 3 parameters to be optimized for: the Gaussian’s center (pulse timing), standard deviation (pulse width) and the peak flow rate. The accuracy of the 4D flow HIKING reconstruction was evaluated by comparing velocity values voxel-by-voxel to laser particle image velocimetry (PIV) data obtained in the same phantom setup.
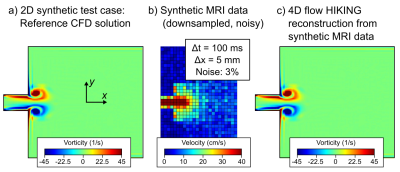
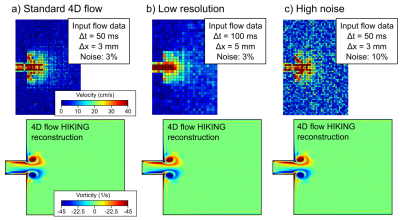
For the synthetic tests, a reference CFD solution in a two-dimensional (2D) geometry was computed (Figure 3). The reference solution was then downsampled (Figure 1b) with added noise to provide simulated MRI data corresponding to a) a consensus 4D flow sequence (7), b) low-resolution conditions, and c) high-noise conditions (Figure 4).
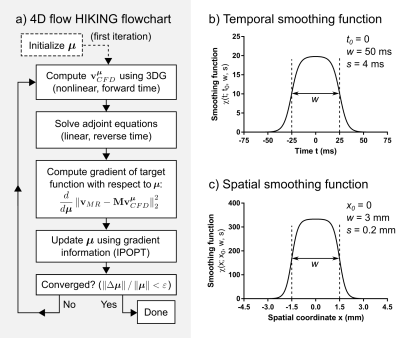
Low-resolution data and a mesh describing the flow domain were used as input to the HIKING framework, which is based on three main components (Figure 5): 1) a target functional measuring the similarity between the MR flow data and CFD solution, linked by the forward model M, 2) the 3DG CFD solver (8), which computes the CFD solution from boundary conditions encoded in the parameter vector µ, and 3) the adjoint equations (8), used to compute the gradient of the target functional. The target functional is then minimized iteratively using the IPOPT library (9) to reconstruct the high-resolution flow that provides a best-fit to the MRI data.
Results
Phantom validation showed very strong agreement between the 4D flow HIKING reconstruction and the laser PIV reference data (Figure 2). Convergence was attained after 40 hours (14 iterations) on a 16-core computer (~640 CPU-hours).
Synthetic test cases showed excellent performance of the 4D flow HIKING framework (Figure 4). Convergence was attained for each synthetic test case after 220 minutes (55 iterations) on a 16-core computer (~60 CPU-hours per case).
Discussion
The phantom validation demonstrates the ability of 4D flow HIKING of reconstructing fully three-dimensional and time-resolved complex flows. The simple model for the inflow consisted of only three parameters, but yielded excellent agreement with laser PIV data. For a more complex flow scenario, e.g. intracranial flow in the Circle of Willis, a more detailed parameterization may be used (at the cost of increased reconstruction time).
The low-resolution two-dimensional synthetic test corresponds to a spatiotemporal undersampling factor of (5 mm/3 mm)2 × (100 ms/50 ms) ≈ 5.6, while still achieving near-perfect flow reconstruction. This highlights the potential of 4D flow HIKING to reduce 4D flow acquisition times and/or increase data quality.
Conclusion
The 4D flow HIKING framework shows excellent flow reconstruction accuracy compared to laser PIV in a phantom setup. Furthermore, synthetic tests show high potential for acceleration and data quality improvements for 4D flow magnetic resonance imaging.Acknowledgements
No acknowledgement found.References
1. Giese D, Wong J, Greil GF, Buehrer M, Schaeffter T, Kozerke S: Towards highly accelerated Cartesian time-resolved 3D flow cardiovascular magnetic resonance in the clinical setting. J Cardiovasc Magn Reson 2014; 16:42.
2. Hsiao A, Lustig M, Alley MT, et al.: Rapid pediatric cardiac assessment of flow and ventricular volume with compressed sensing parallel imaging volumetric cine phase-contrast MRI. Am J Roentgenol 2012; 198:250–259.
3. Rispoli VC, Nielsen JF, Nayak KS, Carvalho JLA: Computational fluid dynamics simulations of blood flow regularized by 3D phase contrast MRI. Biomed Eng Online 2015; 14:110.
4. Funke SW, Nordaas M, Evju Ø, Alnæs MS, Mardal K-A: Variational data assimilation for transient blood flow simulations. arXiv 2016:1607.03013v1.
5. Töger J, Bidhult S, Revstedt J, Carlsson M, Arheden H, Heiberg E: Independent validation of four-dimensional flow MR velocities and vortex ring volume using particle imaging velocimetry and planar laser-Induced fluorescence. Magn Reson Med 2016; 75:1064–1075.
6. Töger J, Kanski M, Arvidsson PM, et al.: Vortex-ring mixing as a measure of diastolic function of the human heart: Phantom validation and initial observations in healthy volunteers and patients with heart failure. J Magn Reson Imaging 2016; 43:1386–1397.
7. Dyverfeldt P, Bissell M, Barker AJ, et al.: 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 2015; 17:72.
8. Zahr MJ, Persson P-O: An adjoint method for a high-order discretization of deforming domain conservation laws for optimization of flow problems. J Comput Phys 2016; 326:516–543.
9. Wächter A, Biegler LT: On the implementation of an interior-point filter line-search algorithm for large-scale nonlinear programming. Math Program 2006; 106:25–57.
Figures