2966
Fully-automated motion correction and probability-based segmentation of myocardial perfusion MRI data1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Imaging Systems - MR, Philips Healthcare, Best, Netherlands, 3Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands
Synopsis
This work presents a fully-automated framework for the pre-processing of free-breathing myocardial perfusion MRI data. Image series are first split into low-rank and sparse components using RPCA. This allows estimation of the deformation fields required to motion correct the image series, in the absence of dynamic contrast enhancement. Once motion corrected, pixels are clustered into anatomically relevant clusters using perfusion-superpixels which groups nearby pixels that have similar time dynamics. A LDA classifier is trained which allows the generation of myocardial probability maps and active contours are fit to the high probability regions to give a delineation of the myocardium.
Introduction
The imaging of the first-pass of a bolus of a Gadolinium-based contrast agent using cardiovascular magnetic resonance (CMR) facilitates the estimation of quantitative values relating to myocardial blood flow.1,2 However, currently clinical evaluation of first-pass CMR is based on visual assessment which is operator-dependent and can be challenging.3 Quantitative analysis can overcome these limitations by providing an automated, more objective and reproducible analysis, leading to increased diagnostic accuracy.4 Despite the fact that quantitative analysis was first proposed over fifteen years ago, it still remains primarily a research tool and it not yet routinely used in clinical practice. One of the major stumbling blocks to be overcome to enable the widespread clinical translation of the technique is the standardisation of the pre-processing of the data.5 In this work, a robust and fully-automated framework is proposed, which allows motion correction and segmentation of free-breathing image series.Methods
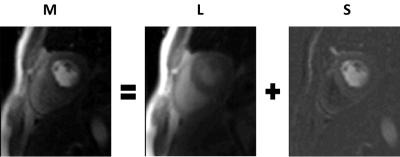
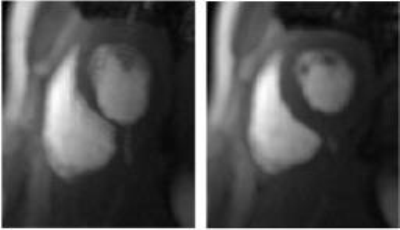
Motion Correction: The local signal intensity changes caused by the dynamic contrast agent inhibit the application of traditional image registration techniques to retrospectively correct for respiratory motion. To counter-act this, a matrix decomposition technique, robust principal component analysis (RPCA), is employed in order to separate the image series into low-rank and sparse components. It has been found that the low-rank component well models the baseline signal and that the sparse component contains the dynamic signal enhancement (see Figure 1).6 Image registration techniques can then be applied to the low-rank component which contains no dynamic contrast. The computed deformation fields are subsequently used to correct the original image series. The image series motion corrected in such a manner lead to clear and well-defined maximum intensity projections (MIPs), which then allows for significantly more accurate segmentation of the myocardium (see Figure 2).
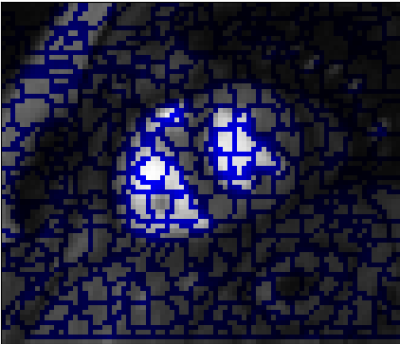
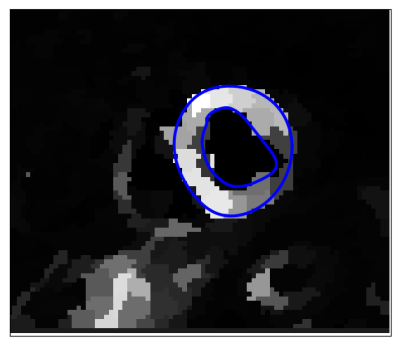
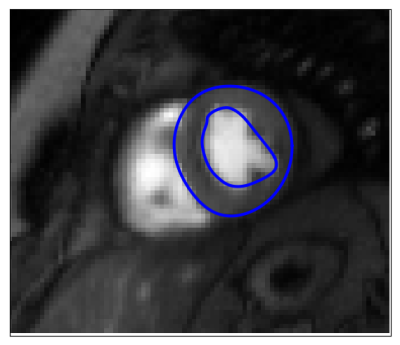
Segmentation: Pixels are clustered into perfusion-superpixels,7 as shown in Figure 3, using simple linear iterative clustering (SLIC)8 to group pixels that are close in both space and intensity in the MIP, as well as having similar time dynamics. This creates anatomically meaningful groups of pixels that already give an over-segmentation of the myocardium. A feature vector is then created for each superpixel based on the principal components of the time-intensity curves for that superpixel and these feature vectors are used to train a linear discriminant analysis (LDA) classifier. Therefore, given a new unseen image series, the classifier can assign a probability that each of its computed superpixels belong to the myocardium. The probability maps, such as Figure 4, locate the myocardium and active contours can then be fit to give a final segmentation. The result of which is shown in Figure 5.
Results
The motion correction was first evaluated by comparing automatically extracted time-intensity curves before and after motion correction to ground-truth curves. The ground-truth curves are manually extracted using a unique segmentation for each frame whereas the automatically extracted curves use only one segmentation as a mask for the whole images series. The mean normalised mean square error with the ground-truth improves from 0.76 (0.8) to 0.50 (0.62) and the mean Pearson correlation coefficient improves from 0.77 (0.25) to 0.89 (0.17) after motion correction (n=60). It was further shown that there is no statistical difference between pharmacokinetic model parameters computed automatically after this motion correction scheme and the ground-truth values which are obtained through manual motion correction. The average Dice coefficient of overlap between the automatically generated myocardial segmentation and expertly drawn contours is 0.70 using 16 image series, with one segmentation failing to converge. 2-fold cross-validation was employed to prevent the classifier learning the segmentation for a rest series using the stress series of the same patient or vice versa.
Conclusion
The described framework is fast, robust and fully-automated. It hence makes quantitative perfusion analysis possible in a matter of seconds with no user-interaction. Work is currently under way to make these methods available online as a web service. It is hoped that this will encourage the standardisation of the quantification process and enable its adoption in clinical practice. The segmentation, however, requires further validation and will benefit from a larger training dataset.Acknowledgements
This work is funded by the King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1)References
- Jerosch-Herold, M., Wilke, N., Stillman, A. E., & Wilson, R. F. (1998). Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Medical Physics, 25(1), 73–84.
- Jerosch-Herold, M., Swingen, C., & Seethamraju, R. T. (2002). Myocardial blood flow quantification with MRI by model-independent deconvolution. Medical Physics, 29(5), 886–897.
- Sammut, E., Zarinabad, N., Vianello, P. F., & Chiribiri, A. (2014). Quantitative Assessment of Perfusion - Where Are We Now? Current Cardiovascular Imaging Reports, 7(7), 1–11.
- Ishida, M., Morton, G., Schuster, A., Nagel, E., & Chiribiri, A. (2010). Quantitative Assessment of Myocardial Perfusion MRI. Current Cardiovascular Imaging Reports, 3(2), 65–73.
- Jerosch-Herold, M. (2010). Quantification of myocardial perfusion by cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance, 12(57), 1–16.
- Hamy, V., Dikaios, N., Punwani, S., Melbourne, A., Latifoltojar, A., Makanyanga, J., Atkinson, D. (2014). Respiratory motion correction in dynamic MRI using robust data decomposition registration - Application to DCE-MRI. Medical Image Analysis, 18(2), 301–313.
- Irving, B., Franklin, J. M., Papiez, B. W., Anderson, E. M., Sharma, R. A., Gleeson, F. V., Schnabel, J. A. (2016). Pieces-of-parts for supervoxel segmentation with global context: Application to DCE-MRI tumour delineation. Medical Image Analysis, 32, 69–83.
- Achanta, R., Shaji, A., Smith, K., Lucchi, A., Fua, P., & Süsstrunk, S. (2012). SLIC superpixels compared to state-of-the-art superpixel methods. IEEE Transactions on Pattern Analysis and Machine Intelligence, 34(11), 2274-2281.
Figures