2962
Rest Perfusion within Chronic Infarctions Depends on Type of Acute Myocardial Infarction: Insights from a Serial MRI Study in Patients1Biomedical Imaging Research Institute, Cedars Sinai Medical Center, Los Angeles, CA, United States, 2Dept of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 3Northern Ontario School of Medicine, Sudbury, ON, Canada
Synopsis
Excessive iron in tissue can impair endothelial function and reduce microcirculatory blood flow. We hypothesized that resting blood flow in chronic hemorrhagic myocardial infarction (hMI) territories, where iron concentration is known to be significantly elevated, would be lower than in non-hMI territories. We studied this in patients with reperfused myocardial infarction using cardiac MRI over a 6-month period following infarction. Mean relative perfusion index of hMIs were significantly lower than non-hMIs. This finding supports the notion that hypoperfusion within hMI territories may be an important pathological contributor to adverse cardiac remodeling commonly observed in patients with hMIs.
Introduction
Pathological accumulation of erythrocyte-derived iron is known to scavenge nitric oxide, causing endothelial dysfunction and reduced microcirculatory blood flow. Given that hemorrhagic myocardial infarctions (hMI) are associated with iron accumulation within infarct territories1, we hypothesized that the resting blood flow in the chronic phase of hMI territories would be lower than in non-hMI territories. We studied this in ST-elevation myocardial infarction (STEMI) patients treated with percutaneous coronary intervention (PCI) and serially followed with cardiac magnetic resonance imaging (CMR).Methods
STEMI patients (n = 17) treated with PCI were recruited for the initial CMR (3-5 days post-intervention) and were followed-up at 6 months with a chronic CMR study (n = 14; 3 subjects were unable to complete the 6-month follow-up). Following localizers T2*-weighted, contrast-enhanced rest perfusion and LGE images were acquired in short-axis orientation on a clinical 1.5T system. Rest perfusion scans were acquired over 3 mid-ventricular slices that were matched to T2* and LGE scans. Based on evaluation of LGE and T2* of acute MI scans, patients were identified to have had hemorrhagic (hMI) or non-hemorrhagic (non-hMI) myocardial infarction. All image analysis was performed using CVI42 (Calgary, Canada). Rest-perfusion images obtained at 6 months were analyzed by first segmenting the LGE images for infarct regions using the mean + 5 SD thresholding method.2,3 The infarct contour was copied and propagated to the T2* images, as well as across the time-resolved perfusion images, adjusting for respiratory motion. A remote region identified on the LGE was also propagated in a similar fashion. Presence of hypo-intense regions within the infarct zone on T2* confirmed hMI. For each subject, Relative Perfusion Index was calculated as: (Perfusion Index of infarct zone) / (Perfusion Index of remote zone), where Perfusion Index is the upslope of signal intensity of the myocardium normalized by the upslope of the signal intensity of the blood pool. A student’s t-test (SPSS, Armonk, NY) was used to determine if the mean Relative Perfusion Index of hMI and non-hMI patients were different. Statistical significance was set at p < 0.05.Results
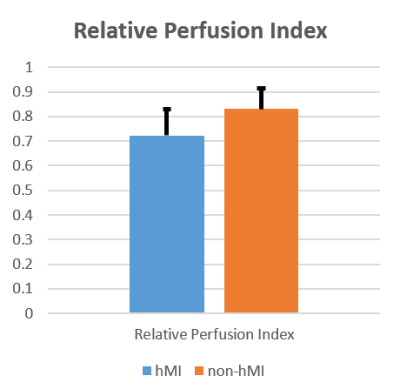
Hemorrhage was evident within acute infarct territories in 6 patients; the remaining 8 patients were classified as non-hMI. Perfusion defects and T2* losses were visible in chronic MI regions in patients with hMIs, but this was evident to a lesser extent in patients with non-hMIs. Representative findings supporting this are shown in Fig. 1. Mean Relative Perfusion Index of hMIs were significantly lower than non-hMIs (0.72 ± 0.11 vs. 0.83 ± 0.08 respectively, p-value = 0.003; Fig.2).Discussion
Although perfusion within chronic infarctions is reduced compared to remote territories, the magnitude of the reduction depends on the type of acute myocardial infarction (hMI vs. non-hMI). This suggests that the pronounced decrease in rest perfusion in the chronic stage of hMI may play a key role in the significantly greater adverse outcomes reported in hMI patients over non-hMI patients.4-9Conclusion
This is the first evidence demonstrating that the rest perfusion in the chronic phase of hMI is lower than non-hMI. While our early findings support the notion that resting perfusion is reduced in chronic hMIs, additional studies are needed to establish the mechanism underlying the rest perfusion defects in chronic hMIs. If confirmed, hemorrhage-mediated hypoperfusion could evolve as a pathological contributor to adverse remodeling observed in hMIs.Acknowledgements
This work was supported in part by a grant from NIH (R01-HL133407).References
1. Kali A, et al. Chronic manifestation of post-reperfusion intramyocardial hemorrhage as regional iron deposition: a cardiovascular MR study with ex-vivo validation. Circulation: Cardiovascular Imaging 2013; 6(2):218-28.
2. Bondarenko O, et al. Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. Journal of Cardiovascular Magnetic Resonance. 2005; 7(2):481-485.
3. Amado LC, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. Journal of the American College of Cardiology. 2004: 44(12):2383-2389.
4. Kali, Avinash, et al. "Persistent Microvascular Obstruction After Myocardial Infarction Culminates in the Confluence of Ferric Iron Oxide Crystals, Proinflammatory Burden, and Adverse Remodeling." Circulation: Cardiovascular Imaging 9.11 (2016): e004996.
5. Rother, Russell P., et al. "The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease." JAMA 293.13 (2005): 1653-1662.
6. Mather AN, Fairbairn TA, Ball SG, Greenwood JP, Plein S. Reperfusion haemorrhage as determined by cardiovascular MRI is a predictor of adverse left ventricular remodelling and markers of late arrhythmic risk. Heart. 2011;97:453–9.
7. Ganame J, Messalli G, Dymarkowski S, et al. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J. 2009;30:1440–9.
8. Nijveldt R, Hofman MB, Hirsch A, et al. Assessment of microvascular obstruction and prediction of short-term remodeling after acute myocardial infarction: cardiac MR imaging study. Radiology. 2009;250:363–70.
9. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nature reviews Cardiology. 2014;11:255-65.
Figures