2960
Pilot Tone Navigation Enables Contactless Prospective Cardiac Triggering: Initial Volunteer Results for Prospective Cine1Siemens Healthcare, Erlangen, Germany, 2Siemens Medical Solutions USA, New York, NY, United States, 3CHUV, Département de Radiologie Médicale, Lausanne, Switzerland
Synopsis
Pilot Tones are a contactless, electromagnetic navigator that offers monitoring of cardiac and respiratory motion independently of the acquisition. Here we present initial volunteer results in utilizing the cardiac Pilot Tone signal to prospectively trigger a segmented cardiac Cine acquisition without the need for ECG.
Introduction
Cardiac MRI depends on accurate quantification of the heart's motion to trigger acquisitions. Four-lead electrocardiograms (ECG) are predominantly used, but suffer from some drawbacks: Patient preparation and lead placement takes time and the ECG signal is prone to artefacts caused by gradient activity and, especially at higher field strengths, the magnetohydrodynamic effect (MHD). Electromagnetic Pilot Tone (PT) navigation, proposed by Speier et al.1 has been found to contain both respiratory and cardiac motion information1,2. We recently demonstrated in a retrospective volunteer study that the cardiac motion signal contains sufficient information3 and could be processed in real-time to enable prospective cardiac triggering. Here, we demonstrate PT triggered prospective segmented cardiac Cine measurements in two volunteers.Methods
All measurements were performed on a 1.5T system (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) on two volunteers (male, 1.62m & 1.7m, 106kg & 80kg, BMI: 40.4 & 27.7). The PT signal was generated by a prototype battery-powered generator (Fig. 1) at a frequency of 64.4MHz, just outside the frequency range of the MR imaging signal but within the extended field of view. The PT signal was received with the scanner’s receiver and extracted and processed on-line in the scanner’s reconstruction environment using a custom written prototype pre-processing step. In order to separate the cardiac signal component $$$s_{card}(t)$$$ we implemented Independent Component Analysis (FastICA4). The ICA unmixing matrix was computed retrospectively for a calibration scan preceding the Cine acquisition. $$$s_{card}(t)$$$ was then processed on-line in the scanner’s image reconstruction environment. To achieve lag-free real-time de-noising we implemented an Extended Kalman Filter (EKF) to fit a parametric model $$$\widetilde{s}_{card}(t)$$$ with seven free parameters to $$$s_{card}(t)$$$.
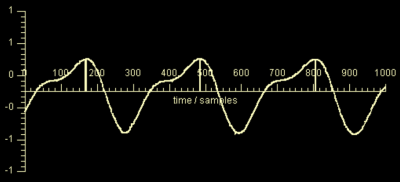
Trigger-points were detected in real-time as maxima of $$$\widetilde{s}_{card}(t)$$$, corresponding to the onset of mechanical systole (See Fig. 2). Upon trigger point detection, a message was sent to the prototype Cine sequence that interpreted it as an ECG R-wave.
To be able to continuously receive the PT signal, we modified the Cine sequence to acquire non-imaging analog-digital conversions, discarded during image reconstruction, while waiting for trigger events. Sequence parameters were: TR=44.8ms & 35.2ms, TE=1.39ms, pixel-bandwidth=975Hz, 12 & 14 segments resulting in an effective PT sampling frequency of $$$f_{PT}=313\textrm{Hz}$$$ and $$$f_{PT}=341\textrm{Hz}$$$,
Results
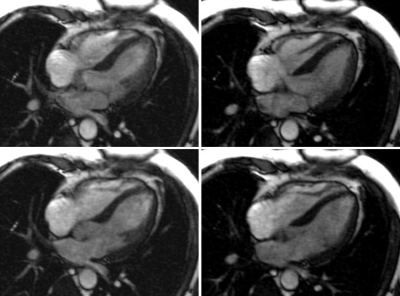
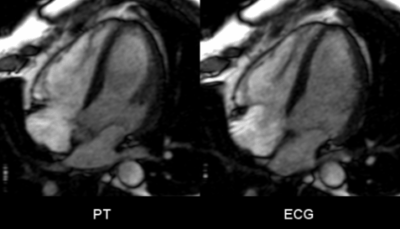
The EKF required approx. 7-12s to settle after sequence start, depending mainly on the accuracy of the initial guess. Figure 3 shows early-systolic and early-diastolic images triggered using PT (left column) vs. conventionally triggered images using ECG (right column). Fig. 2 shows the output of the EKF, $$$\widetilde{s}_{card}(t)$$$ with trigger points indicated. The complete Cine acquisitions are shown in Fig. 4.
We found the unmixing, calculated in the preceding calibration step to decrease in quality over several minutes, resulting in increased offset to $$$s_{card}(t)$$$.
Discussion & Conclusion
Once the ICA has been computed in the calibration step, real-time processing of the cardiac signal in the reconstruction step using an EKF for de-noising is computationally efficient, incurring only minimal delay. Once the EKF has settled, i.e. parameter estimation is stable, the de-noised signal $$$\widetilde{s}_{card}(t)$$$ can be reliably estimated allowing accurate and robust trigger detection. We hypothesize that the cardiac signal is proportional to total cardiac volume. In Fig. 2, mechanical systole, diastole and diastasis, i.e. the phase of relatively little motion during diastole, can be clearly identified.
The resulting Cine images appear visually similar, indicating that PT navigation may be used interchangeably with ECG to prospectively trigger cardiac sequences.
The deterioration in unmixing quality is potentially due to small drifts in the receive system which resulted in large offsets to the cardiac component. After some minutes and in one case where the volunteer shifted position during the exam, calibration had to be repeated to ensure accurate separation of the cardiac component. Alternatively, the ICA solution may be updated iteratively or during breaks between acquisitions.
These encouraging results indicate that PT navigation could be used as a viable alternative to ECG. One potential advantage of PT navigation is that cardiac motion can be measured directly: Using more advanced triggering algorithms, interesting features in the cardiac cycle such as maximum contraction or diastasis could be used for triggering. Another advantage is the possibility to measure cardiac and respiratory motion at the same time. Contrary to MR navigators, PT navigation can be used for any sequence without the need to acquire additional navigator echoes. These encouraging results warrant further research into this method, including patients with arrythmia. Next steps towards clinical usefulness include improvements to the stability of cardiac component separation especially with regard to patient motion and robustness of the EKF de-noising.
Acknowledgements
No acknowledgement found.References
[1] P. Speier et al. "PT-Nav: A Novel Respiratory Navigation Method for Continuous Acquisition Based on Modulation of a Pilot Tone in the MR-Receiver", Proc. ESMRMB 129:97-98, 2015
[2] L. Schröder et al. “A Novel Method for Contact-Free Cardiac Synchronization Using the Pilot Tone Navigator”, Proceedings of the 24th Annual Meeting of the ISMRM (ISMRM 2016), #0410
[3] M. Bacher et al. "Retrospective Evaluation of Pilot Tone Based CardiacTrigger Quality In A Volunteer Cohort", Book of Abstracts ESMRMB 2017 30:360-361, 2017
[4] A. Hyvärinen, ‘‘Fast and robust fixed-point algorithms for independentcomponent analysis’’, IEEE Transactions on Neural Networks, vol. 10, no. 3, pp. 626–634
Figures