2958
Global and segmental cardiac magnetic resonance tissue tracking of hypertrophic cardiomyopathy: How does hypertrophy and fibrosis contribute to myocardial deformation?1Department of Radiology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China, 2Robotics Institute, Shanghai Jiao Tong University, Shanghai, China, 3Department of Radiology, Wayne State University, Detroit, MI, United States, 4Department of Cardiology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China, 5Philips Healthcare, Shanghai, China
Synopsis
In the patients with HCM, subtle LV deformation can be observed and measured clinically before the onset of general LV functional changes. Both hypertrophy and fibrosis influenced the extent of LV deformation. Our study demonstrated the 2D CS as a stable global parameter to assess LV functional and ECV changes. At the segmental level, hypertrophy and LGE (+) antagonistic affected 2D RS and diastolic RSR. Despite the excitement surrounding these pertinent clinical findings, further research is warranted as the mechanism of the phenomenon still needs to be explored.
Introduction
In the early stages of Hypertrophic cardiomyopathy (HCM), the gross systolic and diastolic functions are normal, however the contractility and extensibility of the individual cardiac myocytes are most likely compromised. Both heterogeneous hypertrophy and fibrosis have been shown to contribute to left ventricular myocardial deformation1-4.
The purpose of this study is to investigate global and segmental deformation of HCM with CMR tissue tracking technology and analyze the relationship of heterogeneous hypertrophy and fibrosis using three direction strain parameters. The systolic and diastolic strain of early stage HCM are evaluated separately in this article. Two dimensional and 3D strain analysis were both used to reduce the artifacts from through-plane motion5. Diffuse fibrosis cannot be displayed on LGE and was captured by native T1 and extracellular volume (ECV) changes in order to measure the contribution to segmental strain deformation.
Methods
CMR examinations were performed with a 3.0 Tesla scanner. The images obtained included cine, late gadolinium enhancement (LGE), pre and post T1 sequences. Systolic and diastolic parameters of radial, circumferential, and longitudinal strain (RS, CS, and LS) and strain rate (RSR, CSR, and LSR) were calculated. Global and 16 segmental in 2D and 3D strain parameters were computed. Generalized estimating equation (GEE) models were used to cluster the segments of each patient in order to generalize all segments as if they were measured on one patient.
Results
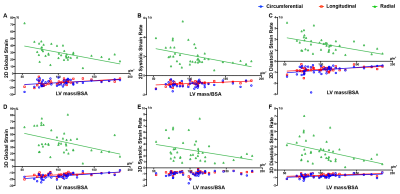
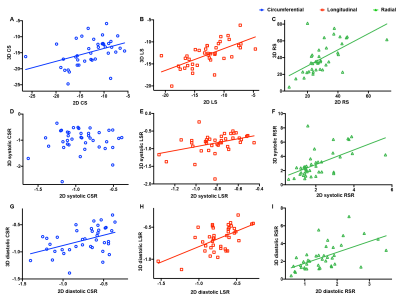
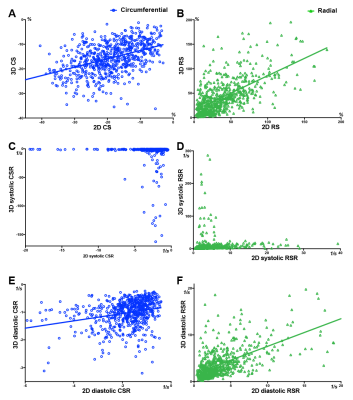
A total of 40 HCM patients were included in this study. The four general parameters of LV function (LV EDV/BSA, LV EDV/BSA, LV EF, LV mass/BSA) relationship with Global native T1 value, ECV, 2D, and 3D strain parameters are displayed in Figure 1. In this study, there is no significant correlation between native T1 value and any classical LV function parameters or strain parameters. However, Global ECV was found to correlate with LV EF and LV ESV/BSA. The relationship of 2D and 3D strain parameters are presented in Figure 2, and Figure 3.
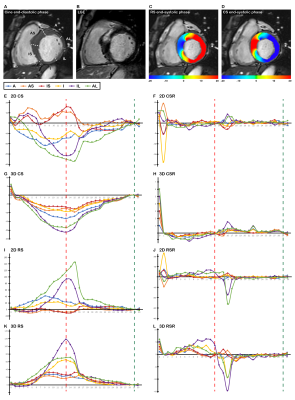
A total of 640 segments were found in 40 HCM patients. The hypertrophied segments mostly concentrated in the anteroseptal, inferoseptal in basal and midventricular levels, in addition to all the segments in apical levels (1-3, 7-9, and 13-16). During the circumferential and longitudinal analysis, segmental hypertrophy (along with LGE presence) correlated with CS, LS and diastolic CSR, LSR, but LGE presence (along with segment thickness ≥ 15mm) was found to only associate with diastolic LSR. The wall thickness ≥ 15mm and LGE (+) were observed antagonistic in 2D RS and diastolic RS.
Discussion
Despite preserved LV EF in HCM patients, subtle changes can be found in the LV resulting in deformation. Several studies have revealed circumferential, longitudinal shortening, and native T1 value and ECV alterations in HCM patients compared to healthy control groups2,6. However, our study fail to show T1 values that assess general LV function or any strain parameters that are seen in HCM patients. Changes in ECV correlated with LV EF and LV ESV/BSA. For this reason, ECV can be considered as a valuable measurement tool to predict HCM development.
Throughout all of our 2D analysis, both LGE presence (along with segment thickness ≥ 15mm) and segmental hypertrophy (along with LGE presence) revealed significant impairment to 2D circumferential and radial parameters. However, in our 3D analysis, circumferential and longitudinal deformation was not significantly affected by both LGE presence and segmental hypertrophy together, albeit radial thickening was still antagonistic affected by the two factors. The variance of 2D and 3D strains, specifically systolic (Figure 3), probably contributed to this result as well. Segmental hypotrophy affected most 3D strains except for the two inconstant systolic parameters. Segmental hypertrophy and LGE (+) antagonistic affected 2D RS and diastolic RSR. The results from our study suggest the presence of LGE (+) and segmental hypertrophy less decreased radial thickening more than the presence of one factor alone. A possible explanation for this finding is that focal LGE probably hinders further hypertrophy but then adds to segmental radial thickening.
Conclusion
In the patients with HCM, subtle LV deformation can be observed and measured clinically before the onset of general LV functional changes. Both hypertrophy and fibrosis influenced the extent of LV deformation. Our study demonstrated the 2D CS as a stable global parameter to assess LV functional and ECV changes. At the segmental level, hypertrophy and LGE (+) antagonistic affected 2D RS and diastolic RSR. Despite the excitement surrounding these pertinent clinical findings, further research is warranted as the mechanism of the phenomenon still needs to be explored.Acknowledgements
Ruo-yang Shi and Bing-hua Chen contributed equally to this work. Lian-ming Wu and Jian-rong Xu are both the corresponding authors for this article.References
- Aletras, A. H.; Tilak, G. S.; Hsu, L. Y.; Arai, A. E., Heterogeneity of intramural function in hypertrophic cardiomyopathy: mechanistic insights from MRI late gadolinium enhancement and high-resolution displacement encoding with stimulated echoes strain maps. Circulation. Cardiovascular imaging 2011, 4 (4), 425-34.
- Nucifora, G.; Muser, D.; Gianfagna, P.; Morocutti, G.; Proclemer, A., Systolic and diastolic myocardial mechanics in hypertrophic cardiomyopathy and their link to the extent of hypertrophy, replacement fibrosis and interstitial fibrosis. The international journal of cardiovascular imaging 2015, 31 (8), 1603-10.
- Kim, E. K.; Lee, S. C.; Hwang, J. W.; Chang, S. A.; Park, S. J.; On, Y. K.; Park, K. M.; Choe, Y. H.; Kim, S. M.; Park, S. W.; Oh, J. K., Differences in apical and non-apical types of hypertrophic cardiomyopathy: a prospective analysis of clinical, echocardiographic, and cardiac magnetic resonance findings and outcome from 350 patients. Eur Heart J Cardiovasc Imaging 2016, 17 (6), 678-86.
- Ghio, S.; Revera, M.; Mori, F.; Klersy, C.; Raisaro, A.; Raineri, C.; Serio, A.; Pasotti, M.; Visconti, L. O., Regional abnormalities of myocardial deformation in patients with hypertrophic cardiomyopathy: correlations with delayed enhancement in cardiac magnetic resonance. Eur J Heart Fail 2009, 11 (10), 952-7.
- Pedrizzetti, G.; Claus, P.; Kilner, P. J.; Nagel, E., Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 2016, 18 (1), 51.
- Almaas, V. M.; Haugaa, K. H.; Strom, E. H.; Scott, H.; Smith, H. J.; Dahl, C. P.; Geiran, O. R.; Endresen, K.; Aakhus, S.; Amlie, J. P.; Edvardsen, T., Noninvasive assessment of myocardial fibrosis in patients with obstructive hypertrophic cardiomyopathy. Heart 2014, 100 (8), 631-8.
Figures