2957
Longitudinal follow-up of endothelial function after ischemia reperfusion injury treated with a novel regenerative therapy by albumin-based DCE MRI1Biomedical Engineering, Biomedical NMR, Eindhoven University of Technology, Eindhoven, Netherlands, 2Biomedical Engineering, Soft Tissue Biomechanics & Tissue Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 3Institute for Complex Molecular Systems, Eindhoven, Netherlands, 4Biomedical Engineering, Organic Chemistry, Eindhoven University of Technology, Eindhoven, Netherlands
Synopsis
Dynamic contrast enhanced (DCE) MRI in combination with gadolinium-labeled albumin enabled longitudinal follow up of a novel hydrogel based regenerative therapy to treat myocardial infarction (MI). The local fractional blood volumes (fBVa measure for microvascular density) and permeability surface areas in the myocardium were increased at day 3 after MI due to the growth factors released from the hydrogel. This increase might indicate angiogenesis, which improves the inflammatory response. At day 7 the vascular density and permeability went back to normal again, which possibly avoid excessive extension of the MI.
Introduction
After a myocardial infarction (MI) the myocardium is irreversibly injured. Angiogenic therapy with vascular endothelial growth factor (VEGF) and insulin-like growth factor-1 (IGF-1) incorporated into a supramolecular ureido-pyrimidinone modified (UPy) hydrogel1 might be a promising approach for repair and regenerate the myocardial vasculature after a MI. In the first 3 days post-MI an adequate inflammatory response is necessary for cleaning debris, while later in infarct healing (from day 7 post-MI) sufficient resolution of the inflammatory response is needed to avoid excessive extension of the ischemic region2. A key factor in the clinical applicability of an angiogenic therapy is to be able to determine the vascular responses at both of these time-points post-MI. Dynamic contrast enhanced (DCE) MRI in combination with gadolinium-labeled albumin (BSA-GdDTPA, 82kDa) enables longitudinal follow up of the local fractional blood volumes (fBV, a measure for microvascular density) and permeability surface area (PS) in the myocardium3. In this study, we evaluate the ability of DCE-MRI to follow the therapeutic effects of a novel growth factor (GF) containing UPy-hydrogel.Methods
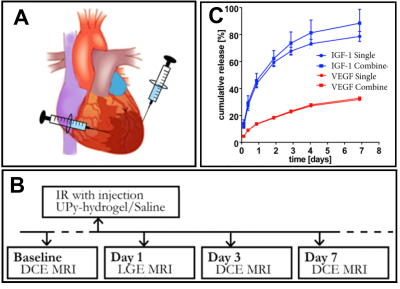
Swiss (OF1) mice with an induced and re-perfused MI were intra-myocardially injected with either saline (n=6) or the VEGF and IGF-1 containing UPy-hydrogel (n=8) (Fig 1A). For both groups, at baseline, day 3 and day 7 post-MI a series of DCE MRI measurements was acquired (Fig 1B). At day 1 after surgery Late Gadolinium Enhanced (LGE) MRI was performed to confirm and locate the infarct. DCE-MRI was performed by intravenous injection of 150μl (100mg/ml) GdDTPA. Prior to administration of the contrast agent, T1-weighted 3D-FLASH images were acquired with a series of variable flip angles (2 ͦ, 5 ͦ, 8 ͦ, 11 ͦ, 13 ͦ ) to determine the native myocardial T1 as described in previous research4. After administration of the contrast agent a series of images was acquired with the same flip angle to determine the post-contrast myocardial T1 over time. The data was post-processed on a voxel-by-voxel basis and a linear fitting algorithm was used to determine the y-intercept (fBV) and the slope (PS/minute) of the GdDTPA concentration in the infarct and remote areas. Furthermore, the ex-vivo release pattern of the GFs was determined by ELISA.Results
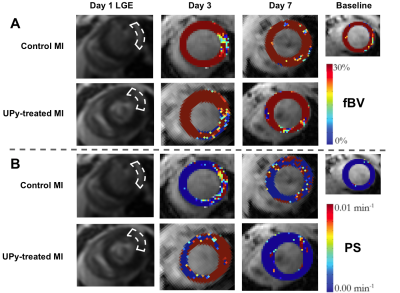
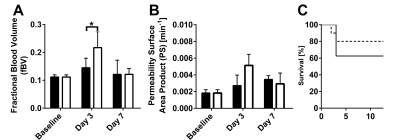
The sustained ex-vivo release of VEGF and IGF-1 were about 23% and 66% at day 3, respectively, and 32.8% and 84.4% at day 7, respectively (Fig 1C). However, the in-vivo release rate can be expected to be higher. DCE MRI revealed a significant increased fBV of the infarct area at day 3 post-MI for group that received the GF containing UPy-hydrogel (Fig 2A and 3A). Also, preliminary results showed a trend towards increased PS at day 3 post-MI for this treated group (Fig 2B and 3B). At day 7 post-MI both fBV and PS appeared to be back at the baseline levels for both groups (Fig 2 and 3A-B). Eventually, the survival rate of the group that received the UPy-hydrogel treatment seemed higher for the first 7 days (Fig 3C), but this was not significant with the current group size.Discussion
DCE MRI was sensitive enough to distinguish an increased fBV due to the UPy therapy at day 3 post-MI. The increased microvascular density (fBV) and the trend of increased permeability (PS) at the infarct due to the UPy therapy at day 3 could have enabled an improved inflammatory response. Normalization of PS and fBV at day 7 post-MI indicated an adequate resolution of inflammatory responses with stabilization of myocardial vasculature. Histology is needed to confirm these findings and long-term follow-up could give insights about possible chronic inflammation and further therapeutic effects due to the hydrogel.Conclusion
DCE-MRI was sensitive enough to longitudinally follow vascular changes induced by a VEGF and IGF-1 containing supramolecular hydrogel therapy for MI treatment. The therapy increased microvascular density and slightly altered permeability in the infarct at day 3 post-MI, indicating enhanced angiogenesis. Also, this treatment enabled adequate resolution of the inflammatory response at day 7 post-MI.Acknowledgements
We would like to acknowledge the great support of Klaas Nicolay in this work.References
1. Bastings MMC, Koudstaal S, Kieltyka RE, et al.: A fast pH-switchable and self-healing supramolecular hydrogel carrier for guided, local catheter injection in the infarcted myocardium. Adv Healthc Mater 2014; 3:70–78.
2. Frangogiannis NG: The Mechanistic Basis of Infarct Healing. Antioxid Redox Signal 2006; 8:1907–1939.
3. Vandoorne K, Vandsburger MH, Jacobs I, et al.: Noninvasive mapping of endothelial dysfunction in myocardial ischemia by magnetic resonance imaging using an albumin-based contrast agent. NMR Biomed 2016; 29:1500–1510.
4. Coolen BF, Geelen T, Paulis LEM, Nauerth A, Nicolay K, Strijkers GJ: Three-dimensional T1 mapping of the mouse heart using variable flip angle steady-state MR imaging. NMR Biomed 2011; 24:154–162.
Figures