2943
Cardiovascular Magnetic Resonance Imaging : a Tool for Non-invasive Absolute Aortic Blood Pressure Estimation1IR4M, Université Paris-Sud, Paris, France, 2IR4M, Université Paris-Sud, Orsay, France
Synopsis
Cardiovascular Magnetic Resonance Imaging (CMRI) is a well-established modality that allows not only non-invasive accurate blood flow quantification but also provides anatomical and biomechanical information about large vessel properties (e.g. aortic wall elasticity and distension) and central hemodynamics. The aim of our study is to use an MR-compatible aortic flow setup including two different elastic phantoms and validate MR-based pressure waveforms predicted by 1D blood flow model against invasive pressure measurements.
Introduction
Cardiovascular Magnetic Resonance Imaging (CMRI) is a well-established modality that allows not only non-invasive accurate blood flow quantification but also provides anatomical and biomechanical information about large vessel properties (e.g. aortic wall elasticity and distension) and central hemodynamics [1]. Particularly, central aortic blood pressure (CABP) has been recognized to be an independent predictor of cardiovascular events as it encodes valuable medical information about the CV system and left ventricle load [2]. However, non-invasive estimation of the absolute and local CABP is still a challenge. Thus, we suggest that combining CMRI-derived parameters to hemodynamic modeling may offer accurate patient-specific prediction of CASBP that enables accurate diagnostic and therapeutic following.
The aim of our study is to use an MR-compatible aortic flow setup including two different elastic phantoms and validate pressure predictions of 1D blood flow model against invasive measurement. To do so, we first assessed phantoms anatomical and biomechanical parameters using CMRI. Additionally, CMR acquisitions provide us with the boundary condition at the inlet and the outlet of the 1D domain. Then, we proceeded to 1D hemodynamic simulations.
Material and Methods
MR-compatible experimental setup :
It consists of a pulsatile programmable flow pump that carries blood mimicking fluid (BMF) from a reservoir to elastic phantoms through stiff hoses (figure 1). Two phantoms with different anatomical and structural properties were used : a straight tube representing the descending aorta and a realistic aorta model. Phantoms were placed sequentially in the center of a 1.5T Phillips Achieva MR magnet. For invasive pressure measurement, each of both silicone models was equipped with two ultrathin fiber optic pressure sensors placed at a proximal and a distal site (with respect to flow direction) as shown in figure 1. In order to obtain realistic presssure ranges, small cascaded tubes were connected at the outlet of the phantoms, thus simulating the in-vivo peripheral resistance.
1D blood flow model :
It provides a simplified description of blood flow interaction with arterial wall displacement and reproduce efficiently the pressure pulse propagation phenomenon. 1D models are derived from the Navier-Stokes equations averaged over vessel cross-sectional area [3]. To take into account the domain dowstream to the 1D model and to avoid non-physiological oscillations on the predicted pressure waves, 1D model is coupled with a 0D lumped parameter model. A first order finite volume scheme was written in Python to solve numerically the hyperbolic system.
CMR acquisitions and 1D model parameters determination :
For each phantom, corresponding 1D model input parameters such as domain length, diastolic cross-sectional area, wall compliance and boundary conditions were determined non-invasively from MR-derived flow and cross-sectional area. Using a 32 channels thorso-cardiac coil, inlet boundary condition was obtained by acquiring through-plane phase-contrast (PC) MR images slightly upstream to the proximal site (figure 2). Another PC-MR acquisition was made downstream to the distal site so as to estimate proximal-distal pulse wave velocity (PWV). Thus, phantom compliance could be deduced from PWV using Bramwell-Hill equation [4]. Moreover, Balanced Steady-State Free Precession Cine images at the proximal site of both phantoms were acquired in order to determine 1D model diastolic cross-sectional area. Both PC and Cine MR images were segmented offline using ARTFUN software.
Validation step consists firstly of comparing MR-based predicted pressure waves at the proximal and distal sites of the straight compliant tube against pressure signals measured invasively at the corresponding anatomical sites. Secondly, we assess the applicability of our model to the realistic aorta phantom.
Results
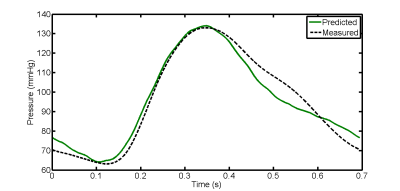
Figure 3 and 4 show MR-based pressure waveforms predicted by our 1D model together with the ones obtained from direct measurements at the proximal site of the straight vessel and of the aorta phatom respectively. Qualitative agreement between computed and measured pressure signals is clearly seen. Room mean square error (RMSE) between both corresponding pressure forms is low suggesting a high quantitative agreement (RMSE = 6.26% (4.99%) for straight tube (realistic aorta phantom)). The same trends were seen at the distal sites.
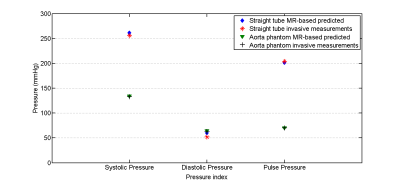
Pressure characteristics indices such as systolic, diastolic, Pulse and mean (not shown) pressures for both predicted and measured pressure are provided in plotted in figure 5.
Discussion and conclusion
Combining CMRI-derveid parameters to hemodynamic simulations offers a non-invasive quantitative tool for accurate assessment of absolute central blood pressure while taking into account geometrical and biomechanical properties of the vessel. This may provide better risk stratification and improve clinical diagnostic.Acknowledgements
NoneReferences
[1] Pohost et al. Handbook of cardiovascular magnetic resonance imaging. CRC Press, 2006.
[2] McEniery et al. Central blood pressure: current evidence and clinical importance. European heart journal, 2014.
[3] Formaggia et al. One-dimensional models for blood flow in arteries. Journal of engineering mathematics, 2003.
[4] Westenberg et al. Bramwell-Hill modeling for local aortic pulse wave velocity estimation: a validation study with velocity-encoded cardiovascular magnetic resonance and invasive pressure assessment. Journal of Cardiovascular Magnetic Resonance, 2012.
[5] De Cesare et al. " ART-FUN": an integrated software for functional analysis of the aorta. Journal of Cardiovascular Magnetic Resonance, 2009.
Figures