2940
Background phase correction in the presence wrap-around artifact: Application in 4D flow imaging1Biomedical Engineering, The Ohio State University, Columbus, OH, United States, 2Cardiovascular MR R&D, Siemens Medical Solutions USA, Inc., Columbus, OH, United States, 3Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH, United States, 4Internal Medicine, The Ohio State University, Columbus, OH, United States
Synopsis
Residual background phase offsets due to eddy-currents limit the accuracy of flow quantification in 4D flow imaging. Commonly utilized polynomial regression of stationary voxels to correct background phase, however, is unreliable in the presence of wrap-around artifact. Here, we present an automated approach to identify and exclude regions of wrap-around from the fit, and validate its effectiveness in phantom and in vivo.
Purpose
Polynomial regression of stationary tissue, commonly used to correct eddy current-induced background phase in phase contrast (PC) MRI, routinely fails in the presence of wrap-around artifact [1]. This problem is of particular concern in 4D flow imaging where wrap-around artifact may occur in both the phase-encode and through-slice dimensions. In this work, we present an extension of our previously proposed outlier rejection scheme [2,3], termed Wrap-around-Rejection (WaR), to exclude wrap-around artifact from the fitting and demonstrate its effect on 4D flow images in phantom and in vivo.Materials and Methods
Theory
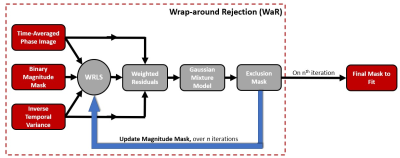
A central aspect of image-based background phase correction involves deciding which voxels should be used and which should be excluded in the polynomial fitting. While pruning and weighting approaches based on the temporal variance are effective in discriminating between regions of flow and stationary tissue, both fail to exclude wrap-around artifact, thereby degrading the correction. WaR, however, provides an automated approach to identify and exclude regions of wrap-around from the fit. The general steps of WaR (Figure 1) are as follows: (1) First, an initial fit is performed using only voxels contained in the central region assumed free from wrap-around artifact. The fitting is computed by our previously proposed Weighted Regularized Least Squares (WRLS) [4]. (2) The weighted residuals between the time-averaged phase image and initial fit are computed over the entire FOV. A strong, localized mismatch, due to wrap-around artifacts, leads to a residual distribution with multiple Gaussian components. (3) A Gaussian mixture model is then fit to the weighted residuals and the mean ($$$μ$$$) and standard deviation ($$$σ$$$) of the primary distribution are extracted and used to define exclusion criteria (4) WRLS is re-performed, excluding voxels defined in (3) from the fit.
Phantom imaging
A stationary oil phantom was scanned on a 1.5 T clinical system (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany). 4D flow images were acquired in an oblique, 144 mm thick slab through the center of the phantom with parameters: TE/TR = 2.69/138.6ms, spatial resolution: 1.2x1.2x3mm3, VENC: 125cm/s, temporal resolution: 138.6ms, FOV: 290x340x144mm3, and IPAT: 2 (GRAPPA). Multiple datasets were collected with different slice oversampling percentages to introduce varying levels of wrap-around artifact in the through-slice direction. In some datasets, the FOV was shifted to introduce additional wrap-around artifact in the phase-encode direction. Background phase was corrected using WRLS and WRLS+WaR. Quality of correction was assessed by two metrics: (1) RMSE was computed over the entire phantom (excluding edges) and (2) the maximum of the mean error within all possible 12x12x12mm3 ROIs inside the phantom was computed to assess worst case performance.
In Vivo imaging
4D flow datasets were acquired in five healthy subjects (age range: 26-63) on a 1.5T clinical scanner (MAGNETOM Aera, Siemens, Healthcare, Erlangen, Germany) using R 7.7 compressive sensing accelerated acquisition. In each subject, a 22-24 slice imaging slab covering the entire aorta was acquired with respiratory navigator gating and prospective cardiac triggering. Parameters for 4D PC-MRI were: TE/TR: (2.27-2.35)/(37.28-37.92)ms, VENC: 150cm/s, spatial resolution: (2.25-2.35)x(2.25-2.35)x(2.5-2.75)mm3, temporal resolution: (37.28-37.92)ms, FOV: (228-285)x(360-380)x(55-66)mm3, and 10% slice oversampling. Background phase was corrected with a 2nd order polynomial using WRLS and WRLS+WaR. For reference, background phase was corrected using WRLS after manually excluding wrap-around artifact from the fit. Root mean-squared error (RMSE) was computed across three volumetric ROIs (15.75x15.75x19.25mm3) in the ascending aorta, aortic arch, and descending aorta. Flow volume from one subject was quantified at five planes using prototype visualization software (Siemens 4D Flow V2.4, Siemens Healthcare, Erlangen, Germany).
Results
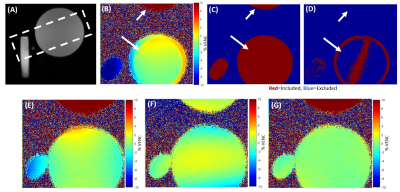
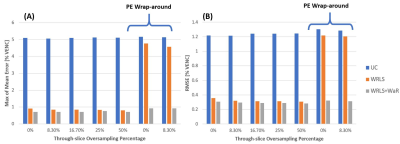
Figure 2B-G demonstrates how WRLS+WaR improved correction compared to WRLS alone. As shown in Figure 3, both maximum of the mean error and RMSE decreased slightly with WRLS+WaR compared to WRLS when only through-slice wrap-around was present. Presence of phase-encode wrap-around degraded the WRLS correction, resulting in substantially less error using WRLS+WaR.
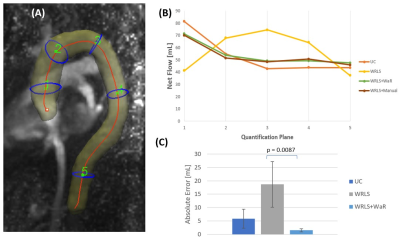
Extensive wrap-around artifact (Figure 4) degraded WRLS correction, resulting an RMSE of over 4% VENC (6 cm/s). Correction with WRLS+WaR brought the RMSE down to 0.55% VENC, or 0.825 cm/s. Figure 5 shows how background phase correction impacts flow quantification in the aorta when extensive wrap-around artifact is present. Net forward flow calculated after WRLS deviated greatly from the reference, performing worse than no correction. The mean of the absolute error with respect to reference (WRLS+manual) from the fives planes decreased significantly from 18.65mL with WRLS to 1.51mL with WRLS+WaR (p=0.0083).
Conclusions
Presence of wrap-around artifact may degrade background phase correction in 4D flow images, limiting diagnostic accuracy of derived flow quantification. In this work, we have presented a wrap-around rejection method robust to such artifact, improving background phase correction, and thereby flow quantification when this artifact is present.Acknowledgements
This work was funded by NIH grant R21EB02227.References
1. Gatehouse et al. JCMR 2012 14:72
2. Pruitt et al. Proc. ISMRM 25 (2017) 2844.
3. Pruitt et al. Accepted SCMR 2018
4. Ahmad et al. Proc. ISMRM. 24 (2016) 2584.
5. Gatehouse et al. JCMR 2010 12:5
Figures