2924
A combined 4D Flow MRI-modelling approach for assessing the subject-specific effects of dobutamine on left ventricular function1Department of Medical and Health Sciences, Linköping University, Linköping, Sweden, 2Center for Medical Image Science and Visualization (CMIV), Linköping University, Linköping, Sweden, 3Department of Biomedical Engineering, Linköping University, Linköping, Sweden, 4Department of Medicine, University of California San Francisco, San Francisco, CA, United States, 5Department of Management and Engineering, Linköping University, Linköping, Sweden
Synopsis
This study applies a previously developed imaging-modelling approach to investigate the subject-specific effects of dobutamine on left ventricular contraction and relaxation patterns in healthy subjects. We created personalized models for nine subjects at rest and under dobutamine stress. The personalized parameter values were in agreement with the effects of inotropy and lusitropy reported in previous studies, and demostrated the anticipated variability in individual responses to dobutamine. With further validation, the given approach has the potential to generate advanced metrics of cardiovascular physiology and pathophysiology that could extend beyond conventional techniques for both diagnosis and optimization of a personalized medical regimen.
Introduction
Dobutamine stress testing is an established method for the diagnosis of ischemic heart disease1. In the healthy heart, dobutamine is expected to induce several effects, including enhanced inotropy, chronotropy and lusitropy2,3. However, the hemodynamic response to dobutamine is, to some extent, unpredictable, and can differ between subjects1,4. Because of these complicating factors, data interpretation would be facilitated by using mathematical modelling. For this reason, we previously developed a method that combines non-invasive measurements, including 4D Flow MRI data, and a lumped-parameter model of the cardiovascular system to obtain personalized model parameters characterizing global hemodynamics5. In this work, we investigate whether this approach can provide subject-specific information on the effects of dobutamine on left ventricular contraction and relaxation patterns in healthy subjects.Methods
Non-invasive measurements were acquired in nine subjects without a known heart condition (mean age: 29±11 years, range 20-56 years, 2 men) at rest and during continuous dobutamine infusion (mean peak dose: 20±7 μg/kg/min, range 10-30 μg/kg/min). Two personalized models, both based on a reduced version of our previous model5, were created for each subject, using the datasets acquired at rest and during dobutamine stress, respectively. Under each physiological condition, morphological cine balanced steady-state free precession (bSSFP) images and 4D Flow MRI data were acquired using a clinical 3T Philips Ingenia scanner (Philips Healthcare, Best, the Netherlands). The acquisition settings are described in Casas et al.5 Heart rate and cuff-based pressure measurements were monitored during the infusion protocol.
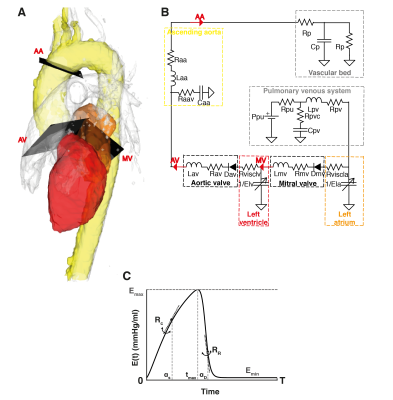
The generic model where parameters are fitted comprises the pulmonary venous system, the left side of the heart, the ascending aorta and a Windkessel model representing the systemic vessels (Figure 1B). The pumping function of the cardiac chambers is described using time-varying elastances6, which relate chamber volume and pressure along the cardiac cycle for the left atrium and the left ventricle, respectively (Figure 1C). Elastance parameters are load independent and well-accepted indexes of cardiac function7,8. Model parameters were personalized based on subject-specific inputs including measurements characterizing the morphology and function of the left ventricle and the aortic valve, as well as 4D Flow MRI-derived volumetric flow waveforms at the mitral valve (MV), the aortic valve (AV) and the ascending aorta (AA) (Figure 1A). The resulting subject-specific parameters based on data at rest and dobutamine stress were compared using a Wilcoxon signed-rank test. A p-value<0.05 was considered significant.
Results
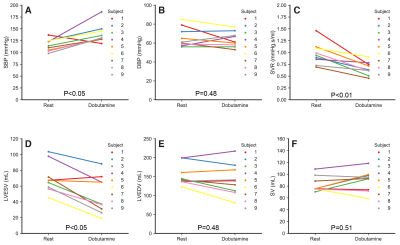
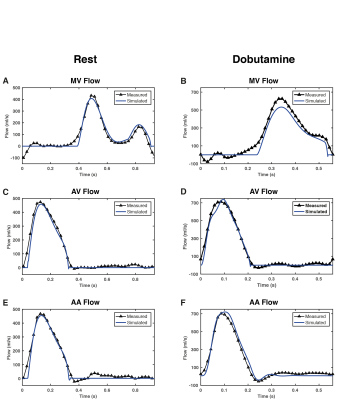
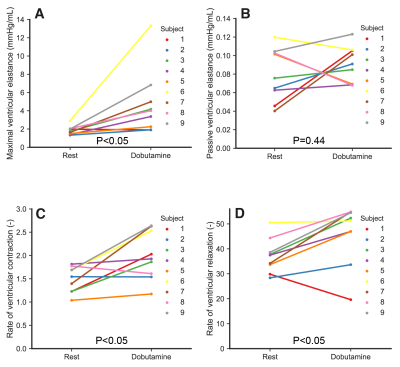
Heart rate increased significantly with dobutamine (66±9 to 105±19 beats/min, p<0.05). Dobutamine-induced changes in indices derived from MRI data and the pressure measurements are shown in Figure 2. SBP increased from to 115.5±12.8 to 139.2±19.9 mmHg (p<0.05), whereas DBP remained unchanged (p=0.48). Subject 1 developed a hypotensive response, with a decrease in SBP from 137 to 119 mmHg. Systemic vascular resistance (SVR) decreased from 0.97±0.23 to 0.66±0.14 mmHg·s/mL (p<0.05). Left ventricular ESV decreased from 70.3±18.9 to 48.9±24 mL (p<0.05). EDV decreased and SV increased in average, but these changes were not significant (p=0.48 and p=0.51, respectively). Figure 3 shows measured and model-based flow waveforms for a representative subject. Dobutamine increased early (E) and late (A) peak flows, from 427±88 to 535±129 mL/s (p<0.05) and from 165±46 to 266±95 mL/s (p<0.01), respectively. The E and A waves merged progressively with increasing heart rates. Dobutamine-induced changes in left-ventricular time-varying elastance parameters are shown in Figure 4. Maximal elastance (Emax,LV) increased from 1.8±0.5 to 4.7±3.6 mmHg/mL (p<0.05), while passive elastance (Emin,LV) decreased non-significantly (p=0.44). The rate of left ventricular contraction (RC,LV) increased from 1.5±0.3 to 2±0.5 (p<0.05) and the relaxation rate (RR,LV) increased, from 37±6.9 to 46±12 (p<0.05), in all subjects except subject 1. The percentage of diastolic duration relative to the cardiac cycle length, based on the estimated time to end-systolic elastance (tmax), decreased from 60±5 to 56±6% (p<0.05).Discussion
The model-based parameters values revealed significant increases with dobutamine in maximal left ventricular elastance and the rates of left ventricular contraction and relaxation. These responses are in agreement with previous studies reporting enhanced inotropy and lusitropy with dobutamine in healthy subjects9-11. In addition, the anticipated variability in individual responses to dobutamine was demonstrable by the subject-specific trends in the measured parameters. Further studies should focus on investigating the added value of these parameters, in a larger group of subjects, for detecting cardiac ischemia and systolic and diastolic ventricular dysfunction in the clinical setting.Conclusion
The imaging-modelling approach provides hemodynamic information that is difficult to obtain solely using an imaging technique, and has the potential to generate advanced metrics of cardiovascular physiology and pathophysiology that could extend beyond conventional techniques for both diagnosis and optimization of a personalized medical regimen.Acknowledgements
No acknowledgement found.References
1. Geleijnse E, Fioretti PM, Roelandt JR. Methodology, feasibility, safety and diagnostic accuracy of dobutamine stress echocardiography. J Am Coll Cardiol 1997;30.
2. Binkley PF, Murray KD, Watson KM, Myerowitz PD, Leier CV. Dobutamine increases cardiac output of the total artificial heart. Implications for vascular contribution of inotropic agents to augmented ventricular function. Circulation 1991;84(3):1210-1215.
3. Parker JD, Landzberg JS, Bittl JA, Mirsky I, Colucci WS. Effects of beta-adrenergic stimulation with dobutamine on isovolumic relaxation in the normal and failing human left ventricle. Circulation 1991;84(3):1040-1048.
4. Blomstrand P, Thulesius O, Wranne B. Cardiovascular effects of dobutamine stress testing in healthy women. Clinical Cardiology 1995;18(11):659-663.
5. Casas B, Lantz J, Viola F, Cedersund G, Bolger AF, Carlhäll C-J, Karlsson M, Ebbers T. Bridging the gap between measurements and modelling: a cardiovascular functional avatar. Scientific Reports 2017;7(1):6214.
6. Suga H, Sagawa K, Shoukas AA. Load Independence of the Instantaneous Pressure-Volume Ratio of the Canine Left Ventricle and Effects of Epinephrine and Heart Rate on the Ratio. Circ Res 1973;32(3):314-322.
7. Schmitt B, Steendijk P, Lunze K, Ovroutski S, Falkenberg J, Rahmanzadeh P, Maarouf N, Ewert P, Berger F, Kuehne T. Integrated Assessment of Diastolic and Systolic Ventricular Function Using Diagnostic Cardiac Magnetic Resonance Catheterization: Validation in Pigs and Application in a Clinical Pilot Study. JACC: Cardiovascular Imaging 2009;2(11):1271-1281.
8. Walley KR. Left ventricular function: time-varying elastance and left ventricular aortic coupling. Critical Care 2016;20(1):270.
9. El-Said E-SM, Roelandt JRTC, Fioretti PM, McNeill AJ, Forster T, Boersma H, Linker DT. Abnormal left ventricular early diastolic filling during dobutamine stress Doppler echocardiography is a sensitive indicator of significant coronary artery disease. Journal of the American College of Cardiology 1994;24(7):1618-1624.
10. Tanimoto M, Pai RG, Jintapakorn W. Normal changes in left ventricular filling and hemodynamics during dobutamine stress echocardiography. Journal of the American Society of Echocardiography;8(4):488-493.
11. Iwakami M, Hashimoto Y, Oniki T, Numano F. Effects of Dobutamine on Left Ventricular Diastolic Performance Are Attenuated in Patients With Systemic Hypertension. The American Journal of Cardiology 1996;78(3):369-372.
Figures