2922
Assessment of Myocardial Fiber Orientation Using Diffusion Tensor Imaging in Patients with Hypertrophic Cardiomyopathy and Its Correlation with Echocardiographic Strain1Yonsei University College of Medicine, Seoul, Republic of Korea, 2Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Cardiovascular Research Center, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 4Department of Bioengineering, University of California, Los Angeles, CA, United States
Synopsis
In hypertrophic cardiomyopathy, myocardial fiber disarray, and interstitial fibrosis interfere with regional systolic myocardial function despite clinically hyperdynamic systolic function. We quantitatively assessed the difference in myocardial fiber orientation between diseased and normal cardiac segments using diffusion tensor imaging. Further, these fiber microstructure were compared to the regional global longitudinal strain to evaluate whether the structure-function relationship changes according to the disease involvement.
INTRODUCTION
The myocardial fiber orientation,1,2 is a crucial determinant of both the mechanical and electrical function of the heart.3,4 Because structural changes of myocardial microstructure usually precede the functional impairment of the heart,4,5 early detection of cardiac remodeling has the potential to improve clinical outcomes.
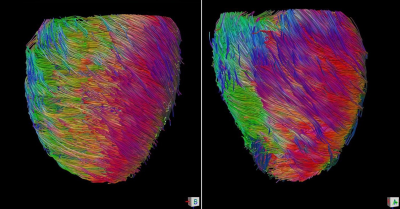
In hypertrophic cardiomyopathy (HCM), myocyte hypertrophy, fiber disarray, and interstitial fibrosis interfere with systolic myocardial function despite clinically hyperdynamic systolic function.6 We assessed the regional difference in myocardial fiber orientation, as assessed by diffusion tensor imaging (DTI),7 in order to describe the relationship between this myocardial fiber architecture and regional systolic function in patients with HCM.
METHODS
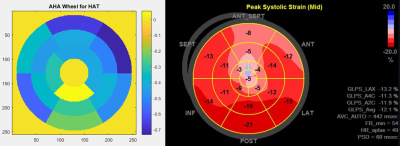
Patients with hypertrophic cardiomyopathy were enrolled and underwent DTI scan and transthoracic echocardiography concurrently.7,8 Using American Heart Association 16 myocardial segmentation, helix angle transmurality (HAT) by DTI and global longitudinal strain (GLS) by echocardiography were calculated for each segment. Segments were classified into non-affected and affected segments, according to the assessment of echocardiography using 15mm as a threshold. HAT and GLS were compared between the groups and correlation between HAT and GLS was calculated.RESULTS
Overall, 7 patients with apical HCM and 3 patients with non-apical HCM (all non-obstructive) were enrolled, and 160 segments are assessed for both HAT and GLS. Among 160 segments, 79 segments were affected by the disease (wall thickness ≥15mm) and 81 segments had normal LV wall thickness (wall thickness <15mm). Affected segments had higher HAT than normal segments (-0.47±0.22 °/% transmural depth [TD] vs. -0.36±0.18 °/%TD, p=0.001), but had decreased GLS compared with normal segments (-12.1±7.2 vs. -14.4±7.0, p=0.037). There was a linear correlation between HAT and GLS in normal segments (R2=0.227, p=0.045), but HAT and GLS had no correlation in diseased segments (p>0.05).DISCUSSION
In HCM, although the hypertrophic regions possessed more greater HAT, the systolic function was decreased compared with normal segments and the correlation between the fiber orientation and systolic function was lost. This result implies that the segmental hypertrophy is not necessarily linked to the contractile function of the myocardium in HCM patients.CONCLUSION
Regional assessment of myocardial fiber orientation using DTI could offer a unique approach to quantify regional involvement of HCM.Acknowledgements
No acknowledgement found.References
1. Streeter DD, Spotnitz HM, Patel DP, Ross J, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circulation research 1969;24:339-47.
2. Stephenson RS, Agger P, Lunkenheimer PP, et al. The functional architecture of skeletal compared to cardiac musculature: Myocyte orientation, lamellar unit morphology, and the helical ventricular myocardial band. Clinical Anatomy 2015.
3. Hooks DA, Trew ML, Caldwell BJ, Sands GB, LeGrice IJ, Smaill BH. Laminar arrangement of ventricular myocytes influences electrical behavior of the heart. Circulation Research 2007;101:e103-e12.
4. LeGrice I, Takayama Y, Covell J. Transverse shear along myocardial cleavage planes provides a mechanism for normal systolic wall thickening. Circulation Research 1995;77:182-93.
5. Arts T, Prinzen FW, Snoeckx L, Rijcken JM, Reneman RS. Adaptation of cardiac structure by mechanical feedback in the environment of the cell: a model study. Biophysical Journal 1994;66:953-61.
6. Carasso S, Yang H, Woo A, et al. Systolic myocardial mechanics in hypertrophic cardiomyopathy: novel concepts and implications for clinical status. Journal of the American Society of Echocardiography 2008;21:675-83.
7. Nguyen C, Fan Z, Sharif B, et al. In vivo three‐dimensional high resolution cardiac diffusion‐weighted MRI: A motion compensated diffusion‐prepared balanced steady‐state free precession approach. Magnetic resonance in medicine 2014;72:1257-67.
8. Serri K, Reant P, Lafitte M, et al. Global and regional myocardial function quantification by two-dimensional strain. Journal of the American College of Cardiology 2006;47:1175-81.
Figures