2921
Understanding the material behaviour of ex-vivo porcine hearts using MR-Elastography and Rheology1Biomedical Engineering, King's College London, London, United Kingdom, 2Institute of Biomechanics, Graz University of Technology, Graz, Austria
Synopsis
Myocardial stiffness has been shown to correlate with heart disease, nevertheless, reliable stiffness estimates are hindered by the remarkably complex behaviour and function of the heart muscle. In this work, we use MR-Elastography and rheological experiments to obtain a better understanding of the myocardial material behaviour. MR-Elastography and cyclic shear tests are performed on ex-vivo porcine hearts, under varying frequencies. Our results demonstrate important tissue properties and highlight the viscoelastic properties of the myocardium which are often neglected. Improving our understanding of the interlinked material properties of the heart is a critical step towards the accurate prediction of myocardial stiffness.
Introduction
Myocardial stiffness has been described as an important indicator of the health of the heart muscle1, with the potential of assisting in disease diagnosis and treatment planning. Significant research effort has been devoted to assessing and studying myocardial stiffness through patient-specific modelling2, pressure-volume analysis, and mechanical testing3. However, the use of myocardial stiffness as a clinical index remains challenging, predominantly due to the intricate nature of the heart tissue, presenting nonlinear, hyperelastic, anisotropic and viscoelastic material behaviour. Obtaining a better understanding of these interlinked properties is a critical step towards accurately assessing heart stiffness.
In this work, we attempt a closer look at the heart’s behaviour using MR-Elastography (MRE), an imaging technique utilising propagating shear waves to estimate material properties4,5. MRE is performed on ex-vivo porcine hearts, at several wave frequencies, creating a platform for understanding the frequency response of the myocardial tissue and its –often neglected– viscoelastic properties. Complementary information on the myocardial material behaviour is provided by multi-frequency cyclic shear tests performed on excised specimens of porcine hearts. Linking MRE with rheology, we can obtain a more complete picture of the heart’s material properties.
Methods
MRE and rheology experiments were performed on 8 ex-vivo porcine heart samples. The hearts were rapidly excised and immersed in a Prosphate Buffered Saline (PBS) and 2,3-Butanedione monoxime (BDM) solution. BDM is known to block cross-bridge activity thus preventing the contraction of the muscle. All experiments were done within 24 hours, to avoid significant deterioration of the tissue.
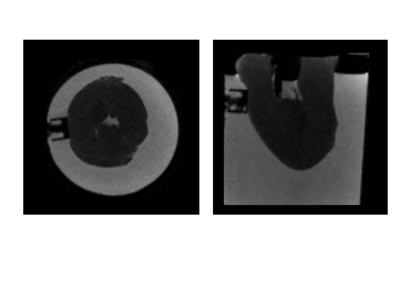
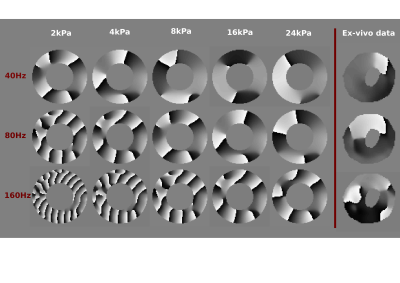
The MRE experiments were performed on excised left ventricles (LV) in a 3T Philips Achieva scanner. An electromagnetic transducer was used to propagate waves into the hearts, at three driving frequencies (40, 80, 160Hz). At each driving frequency, high resolution T2-weighted anatomical images (Figure 1) as well as MRE data6 were acquired (Figure 2).
Computational simulations were run, to assist with interpreting the relation between the wave behaviour and material stiffness. Specifically, the propagation of waves of varying frequencies (40, 80 and 160Hz) was simulated on a cylindrical geometry of similar size as a porcine LV, replicating the MRE experiments. Five stiffness values were employed for each frequency (2, 4, 8, 16, 24 KPa), creating a comprehensive map of frequency-stiffness response (Figure 2). In all simulations viscosity was set to one third of the assumed stiffness value.
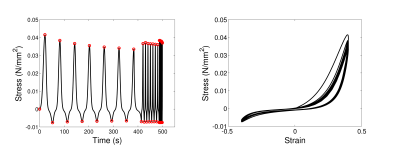
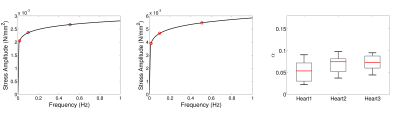
Rheological experiments were performed on small (8x8x8mm3) cubic specimens of the porcine hearts. The specimens were subjected to cyclic shear, with a varying cyclic frequency. Shear was applied on two perpendicular directions (Figure 3), at two different strain magnitudes (0.3, 0.4). The tissue frequency response was approximated by a power-law between frequency (F) and stress amplitude (SA, SA = cFα). The power α was estimated through least-square fitting, using data in both shearing directions in three hearts (Figure 4).
Results and Discussion
The rheological shear data in Figure 3 highlight important material properties of the myocardial tissue. The nonlinear behaviour can easily be deduced in the stress-strain response, while the observed hysteresis loop and the relaxation of the stress amplitude with time in each frequency regime suggest the viscoelastic nature of the myocardium. Additional evidence is provided by the increase in stress amplitude with increasing frequency (Figures 3 and 4). The values of α are consistent across the three hearts examined, and suggest a weak but not negligible frequency response.
The dependence of the material behaviour on frequency can also be seen in the MRE data in Figure 2. Interestingly, the acquired wave data present large wavelengths, suggesting high stiffness for the ex-vivo hearts. This finding is not in accordance with the low literature values (approximately 4kPa 7,8) reported for MRE-derived cardiac stiffness. Our MRE results, however, agree with our simulation results which, for all frequencies, indicate that stiffness values of 16kPa or higher are required to produce wavelengths similar to the ones observed in ex-vivo data.
Conclusions
The presented rheological and MRE experiments offer a comprehensive description of myocardial material properties, highlighting the viscoelastic nature of the cardiac tissue which is most commonly neglected. Improving our understanding of the remarkably complex mechanical behaviour of the heart is an essential step towards the reliable estimation of myocardial properties and their potential use as clinical biomarkers.Acknowledgements
Authors would like to acknowledge funding from the EPSRC (EP/N011554/1 and EP/R003866/1). The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Wang J, Nagueh F. Current perspectives on cardiac function in patients with diastolic heart failure. Circulation 2009; 119:1146-1157.
2. Hadjicharalambous M, Asner L, Chabiniok R, et al. Non-invasive model-based assessment of passive left ventricular myocardial stiffness in healthy subjects and in patients with non-ischemic dilated cardiomyopathy. Ann. Biomed. Eng. 2017; 45(3):605-618.
3. Sommer H, Schrief A, Andrä M, et al. Biomechanical properties and microstructure of human ventricular myocardium. Acta biomaterialia. 2015;24:172-192.
4. Sinkus R, Siegmann K, Xydeas T, et al. MR Elastography of Breast Lesions: Understanding the Solid/Liquid Duality Can Improve the Specificity of Contrast-Enhanced MR Mammography. Magn. Reson. Med. 2007; 58(6):1135-1144.
5. Huwart L, Sempoux C, Vicaut E, et al. Magnetic Resonance Elastography for the Noninvasive Staging of Liver Fibrosis. Gastroenterology. 2008; 135(1):32-40.
6. Garteiser P, Sahebjavaher R, Ter Beek, et al. Rapid acquisition of multifrequency, multislice and multidirectional MR elastography data with a fractionally encoded gradient echo sequence. NMR in biomedicine. 2013;26:1326-1335.
7. Arunachalam S, Arani A, Baffour F, et al. Regional assessment of in vivo myocardial stiffness using 3D Magnetic Resonance Elastography in a porcine model of myocardial infarction. Magn. Reson. Med. 2017.
8. Mazumder R, Schroeder S, Mo X, et al. In vivo magnetic resonance elastography to estimate left ventricular stiffness in a myocardial infarction induced porcine model. JMRI. 2017;45(4):1024-1033.
Figures