2910
Simultaneous Multi-Slice Gradient Echo Spin Echo EPI (SMS-GESE-EPI) enables simultaneous cardiac T2 and T2* imaging and mapping across six slices within a single heartbeat1Department of Radiology, University of Groningen, University Medical Center Groningen, Groningen, Netherlands, 2A.A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Cardiovascular Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States, 4Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 5Oslo University Hospital, Oslo, Norway, 6Department of Nuclear Medicine and Molecular Imaging, University of Groningen, University Medical Center Groningen, Groningen, Netherlands, 7Department of Photonic Imaging, University of Twente, Enschede, Netherlands, 8Medical Imaging Centre of Southwest Finland, Turku University Hospital, Turku, Finland, 9Department of Radiology, Harvard Medical School, Boston, MA, United States, 10Division of Health Sciences and Technology, Harvard-MIT, Cambridge, MA, United States
Synopsis
Cardiac T2* and T2-based techniques suffer from variabilities introduced by acquisition over multiple heartbeats and breath holds. We demonstrate the use of a dual-echo SMS-GESE-EPI sequence that can simultaneously provide T2*- and T2-weighted images from six slice locations within a single heartbeat and breath-hold. Introduction of 5-echos also enabled dynamic T2*- and T2-mapping per heartbeat within a breath-hold. These dynamically acquired T2*- and T2-maps remained stable over ten heartbeats. Several applications might benefit from these modified GESE sequences, such as BOLD measurements and vessel architecture imaging of the myocardium.
Introduction
The onset and progression of cardiomyopathies is often difficult to determine with conventional CMR. T2*- and T2-based sequences offer new opportunities for non-invasive identification of myocardial tissue characteristics1. The major drawback of most T2*- and T2-based techniques is the acquisition over multiple heartbeats and breath holds. In particular, there is significant variability in standard T2*- and T2-mapping2, where the data becomes motion prone. It is also not possible to dynamically acquire multiple slices per heartbeat, which imposes limits on dynamic measurements of myocardial BOLD3. Furthermore, novel imaging techniques such as vessel architecture imaging4 are not translatable to the heart through these standard approaches. In this work, we provide a proof-of-concept application of the gradient echo spin echo EPI (GESE-EPI) to cardiac, which can simultaneously provide T2*- and T2-weighted images5. Although this sequence is encoding intensive, the advent of high-channel coils and simultaneous-multi-slice (SMS) acquisition now makes it possible to perform such acquisition in an efficient manner, with significant slice-coverage per heartbeat. Here, we demonstrate the use of the dual-echo SMS-GESE-EPI sequence at six slice locations within a single heartbeat. We also show that acquisition of five echoes (also published as SAGE6) enables dynamic time series of T2*- and T2-maps of six slices within one heartbeat and breath-hold.Methods
SMS-GESE-EPI was applied to a healthy volunteer on a 3T Prisma Siemens MRI with a 18ch body and 32ch spine coil with the following parameters: ECG triggering at end diastole, acceleration factor=12 (RinplanexRzoomxMB=2x3x2), res=2.77x2.77x7mm, 6 slices, motion-robust FLEETed GRAPPA-training acquisition7. An asymmetric saturation pulse was used to achieve sharp saturation and decrease the FOVPE to 37.5% (FOV=127x350x92mm). Two separate acquisitions were performed: 1) a dual-echo GESE with TEs=11/60ms, TR=320ms, and 2) a 5-echo GESE with TEs=9/21.61/34.24/46.86/59.48ms, TR=400ms. The resulting images from the 5-echo GESE were used for T2*- and T2-mapping per heartbeat by using a four-parameter fit6.
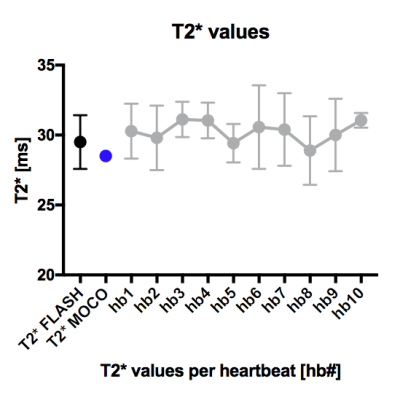
For comparison, a single slice was acquired with standard TSE and FLASH based T2*- and T2-weighted sequences. Furthermore, a TSE T2-map (TEs=5.5/22/38/60/82ms, TR=1784, FOV=208x256mm, res=1.33x1.33x5mm, FA=180) and FLASH T2*-map (TEs=5/9.4/15ms, TR=700, FOV=276x340mm, res=1.33x1.33x5, FA=30) were acquired. The dual-echo sequence was evaluated by visually comparing to standard methods, as well as by investigating septal intensity changes over time. Manually drawn ROIs in the septum were used to compare the T2*- and T2-values from the GESE based maps with the TSE and FLASH based maps. Furthermore, the dynamically acquired GESE T2*- and T2-maps were statically analyzed to confirm stability over time.
Results
T2*- and T2-weighted images were simultaneously acquired across six slices per heartbeat using the dual-echo GESE sequence, resulting in ten of such measurements per breath-hold. As expected, the benefits in speed and coverage of GESE came at a cost of lower image-SNR when compare to the TSE and FLASH images (Fig.1). Also, over the ten dynamic images, the signal intensity in the septum changed by 14-20% (Fig.1E). The 5-echo GESE T2-maps (Fig.2A) gave similar T2-values as the TSE T2-map (Fig.2B) and remained consistent over all dynamic measurements (Fig.3). The GESE T2*-maps (Fig.2C) gave similar T2*-values as the FLASH T2*-maps (Fig.2D) and also remained consistent over all measurements (Fig.4).Discussion
The SMS-GESE-EPI enabled simultaneous and dynamic acquisition of T2*- and T2-weighted images, and T2*- and T2-maps of six slices per heartbeat, which can improve several current and new myocardial tissue characterization applications. The intensity change of the T2*- and T2-weighted images over the dynamic acquisition was larger than expected, but likely due to incomplete T1 recovery between heartbeats coupled with TR variability. Variation due to T1 could be mitigated by optimizing the flip angle, post-processing correction with native T1 maps, or using a 5-echo GESE sequence to derive T2*- and T2-maps directly. The 5-echo SMS-GESE-EPI resulted in T2*- and T2-maps that were stable over time with values that correspond with the TSE and FLASH based maps and previous studies8. However, the field inhomogeneity and slice thickness in cardiac T2*- and T2-imaging causes a strong signal loss at the lateral wall, which is commonly solved by restricting analysis to a septal ROI. Finally, the acquisition would benefit significantly from incorporation of black blood preparation, which is one of our future directions.Conclusion
The GESE-EPI with SMS was successfully applied to the heart and enabled dynamic myocardial T2*- and T2-imaging of multiple slices per heartbeat. Introduction of 5-echo acquisition also enabled dynamic T2*- and T2-mapping. These modified GESE sequences could potentially be used for several applications, such as BOLD and vessel architecture imaging of the myocardium.Acknowledgements
This work was supported in part by NIH research grants: R01EB020613, R01EB019437, R24MH106096, P41EB015896, and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, S10RR023043References
1. Captur G, Manisty C, Moon JC: Cardiac MRI evaluation of myocardial disease. Heart 2016; 102:1429–1435.
2. de Roquefeuil M, Vuissoz PA, Escanyé JM, Felblinger J: Effect of physiological Heart Rate variability on quantitative T2 measurement with ECG-gated Fast Spin Echo (FSE) sequence and its retrospective correction. Magn Reson Imaging 2013; 31:1559–1566.
3. Nagao M, Yamasaki Y, Kawanami S, et al.: Quantification of myocardial oxygenation in heart failure using blood-oxygen-level-dependent T2* magnetic resonance imaging: Comparison with cardiopulmonary exercise test. Magn Reson Imaging 2017; 39:138–143.
4. Emblem KE, Mouridsen K, Bjornerud A, et al.: Vessel Architectural Imaging Identifies Cancer Patient Responders to Anti-angiogenic Therapy. Nat Med 2013; 19:1178–1183.
5. Cornelius Eichner, Kourosh Jafari-Khouzani, Stephen Cauley, Himanshu Bhat, Pavlina Polaskova, Ovidiu C. Andronesi, Otto Rapalino, Robert Turner LL, Wald, Steven Stufflebeam and KS: Slice Accelerated Gradient-Echo Spin-Echo Dynamic Susceptibility Contrast Imaging with Blipped CAIPI for Increased Slice Coverage. Magn Reson Med 2014; 72:770–778.
6. Schmiedeskamp H, Straka M, Newbould RD, et al.: Combined spin- and gradient-echo perfusion-weighted imaging. Magn Reson Med 2012; 68:30–40.
7. Chapman B, Turner R, Ordidge RJ, et al.: Real‐time movie imaging from a single cardiac cycle by NMR. Magn Reson Med 1987; 5:246–254.
8. Roy C, Slimani A, de Meester C, et al.: Age and sex corrected normal reference values of T1, T2 T2* and ECV in healthy subjects at 3T CMR. J Cardiovasc Magn Reson 2017; 19:72.
Figures