2907
Iron-Ceroid Complex From Apoptotic Siderophage-Derived Foam Cells Promotes Perpetual Macrophage Ingress and Localized Edema Formation in Hemorrhagic Myocardial Infarctions: Histopathology and Immunohistochemistry Findings to MRI Correlates1Cedars-Sinai Medical Center, Los Angeles, CA, United States
Synopsis
The capacity of macrophages (MΦ) to oxidize LDL, produce ceroid (CR), and transform into foam cells (FC) is enhanced following erythrophagocytosis. During the process of FC formation, part of hemoglobin-derived iron forms a complex with CR. CR is cytotoxic; and over time, it can lead to FC apoptosis. Release of CR from apoptotic FC into the surrounding tissue may cause dysfunction and apoptosis of newly invading MΦ. Given that lipid and iron deposits within hemorrhagic MI (hMI) typically colocalize in the infarct periphery, we hypothesized that CR from apoptotic FC promotes perpetual MΦ ingress and localized edema formation in hMI.
INTRODUCTION
Following red blood cell phagocytosis, iron-laden macrophages (siderophages) tend to oxidize surrounding LDL, accumulate cholesterol, produce ceroid-type lipopigment and transform into foam cells. During the process of foam cell formation, part of hemoglobin-derived iron forms a complex with developing ceroid. Ceroid is undegradable and cytotoxic, and over time, it can lead to lysosome destabilization, leakage of lysosomal contents, and finally apoptosis of foam cell. Release of ceroid from apoptotic foam cells into the surrounding tissue thus constitutes a "death zone" containing toxic materials that may cause dysfunction and apoptosis of newly invading macrophages. This can further lead to a progressive accumulation of dead or dying phagocytic cells incapable of resolving the lesion but still capable of releasing cytokines, thereby promoting the self-perpetuating and amplifying loop of phagocyte ingress and apoptosis.
Recent studies have shown that iron deposits within hemorrhagic MIs facilitate perpetual recruitment of macrophages throughout chronic phase of MI. The lack of efficient clearance of iron within hemorrhagic MIs implies that macrophages cannot function properly within the environment of abnormal iron deposition. We hypothesized that ceroid from apoptotic siderophage-derived foam cells drives the perpetual increase in macrophage recruitment and expansion of “death zone” in hemorrhagic MI.
METHODS
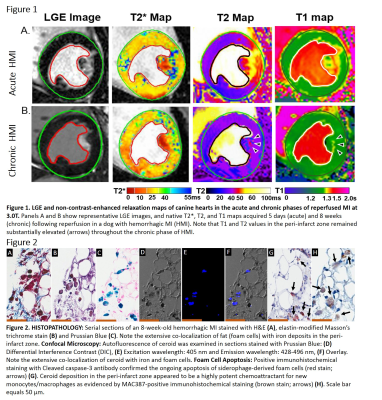
Canines (n=10) were subjected to 3-hour occlusion of the LAD artery, followed by reperfusion. All dogs underwent native T1, T2, T2* and LGE MRI (covering the whole left ventricle) in a 3T clinical MRI system (MAGNETOM Verio, Erlangen, Siemens Healthcare) on day 5 and week 8 post-MI. T1 maps were acquired with MOLLI (8 inversion times [TI] with 2 Look-Locker cycles of 3+5 images, minimum TI=120ms, TI increment=80ms, flip angle=35°, and readout bandwidth=1371 Hz/pixel). T2 maps were acquired using multiple T2 preparations (T2 preparation times=0, 24, and 55ms) with balanced SSFP readouts (flip angle=70°, readout bandwidth of 1371 Hz/pixel). T2* maps were acquired from multi-gradient recalled acquisitions (TR=12ms, 6 TEs=2.0ms–9.5ms with ΔTE=1.5ms, flip angle=10° and readout bandwidth of 930Hz/pixel). LGE image were acquired with PSIR reconstruction (balanced SSFP readouts with TR/TE=3.42/1.47ms; inversion time was optimized to null viable myocardium; readout bandwidth=586 Hz/pixel; flip angle=20o). LGE images were acquired 10 minutes after intravenous injection of 0.2 mmol/kg gadopentetate dimeglumine (Magnevist, Bayer Healthcare, US). Voxel size for all acquisitions was fixed to 1.5×1.5×8mm3. Mean segmental T1, T2 and T2* values were measured using cvi42 (Circle Cardiovascular Imaging Inc.). After MRI on week 8 post-MI, animals were sacrificed and hearts were explanted for histopathological analysis. Sections stained with Prussian Blue were examined for autofluorescence of ceroid under confocal microscope. Apoptosis of siderophage-derived FCs was detected using cleaved caspase-3 antibody. Persisting phagocyte ingress was detected by MAC387 antibody (marker of newly recruited macrophages).RESULTS
All dogs exhibited intramyocardial hemorrhage on day 5 post-MI. Peripheral zone of hemorrhagic MIs exhibited significantly elevated T1 and T2 values relative to the infarct core and remote myocardium, suggestive of edema/inflammation and localized fat deposition (Figure 1). Histopathological evaluation demonstrated the enhanced regional colocalization of iron, ceroid, apoptotic FCs and newly recruited macrophages (Figure 2).DISCUSSION
Our findings suggest that iron-ceroid complex from apoptotic siderophage-derived FCs promotes localized macrophage ingress and expansion of “death zone” in hemorrhagic MIs. These findings may explain the unresolved edema observed in hemorrhagic MIs 8 weeks post MI.CONCLUSION
We conclude that iron-ceroid complex is a key driver of prolonged localized inflammation and accelerated adverse left ventricular remodeling in hemorrhagic MIs.