2902
Comparison of GRE, SSFP, and CINE CMR Acquisitions for Measuring Magnetization Transfer at 3T1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States
Synopsis
We developed and compared multiple magnetization transfer (MT) pulse sequence strategies to characterize myocardial fibrosis without the need for gadolinium contrast at 3T. We demonstrated in an initial study of 4 healthy volunteers that a free-breathing, single-shot GRE is the most effective technique for producing high quality myocardial MT ratio maps. We will continue refining and investigating this sequence as a method for quantifying both focal and diffuse fibrosis in patients with heart failure.
Introduction
Both focal and diffuse myocardial fibrosis occur in a number of cardiac pathologies. Late gadolinium-enhanced (LGE) CMR detects focal myocardial fibrosis. Extracellular volume fraction (ECV), as assessed by T1 mapping pre and post contrast, can quantify myocardial extracellular expansion in the presence of fibrosis. The presence of LGE and/or increased ECV are diagnostically important, and are markers of poor prognosis1–3. However, administration of gadolinium contrast is contraindicated in patients with severe renal dysfunction due to nephrogenic systemic fibrosis (NSF)4,5. Furthermore, there is growing concern regarding gadolinium deposition in patients undergoing repeated MRI examinations. As such, there is a clinical need for contrast-free alternatives for myocardial tissue characterization in heart failure patients.
Magnetization transfer (MT) imaging is a contrast-free technique that may be sensitive to both focal and diffuse fibrosis. Previous ex-vivo rat model, in-vivo mouse, and in-vivo human acute myocardial infarction studies have shown that MT ratio (MTR) maps correlate to LGE with high sensitivity and specificity6–8. Another study at 1.5T demonstrated an increased MT in patients with chronic kidney disease using an SSFP cine technique with 5 degree and 45 degree flip angles to generate the MTR maps9. However, to date, there is limited clinical information on MT imaging in chronic infarction and diffuse fibrosis. We aim to develop and optimize an MT sequence to characterize both diffuse and focal fibrosis without the need for gadolinium contrast.
Methods
We developed a number of MT-weighted pulse sequence strategies as shown in Figure 1 and evaluated them on 4 healthy volunteers with a 3T MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). The MT preparation consisted of 5 Gaussian pulses with an off resonance frequency of 800 Hz, flip angle of 1500°, and pulse duration of 10 ms. MT and Non-MT images were acquired in separate acquisitions. For single-shot acquisition strategies, images were acquired every other heartbeat. The separate MT and reference (Ref) datasets were motion corrected using the symmetric normalization algorithm10 prior to deriving the MT maps. For comparison to a previously described cine-SSFP MT technique, MTR maps were created from cine-SSFP images acquired with different MT weights (flip angles 5° and 45°)9. MTR maps were calculated as
$$ MTR = 100 \times \frac{MT-Ref}{Ref} $$.
ROIs were then drawn on the myocardium and in a noise region outside the body for signal-to-noise (SNR) assessment. An ROI was drawn on the MTR maps to quantify myocardial MTR. Data were compared using a repeated measures ANOVA and with multiple comparisons test. A blinded cardiologist scored the 6 imaging techniques (1-worst, 5-best).
Results
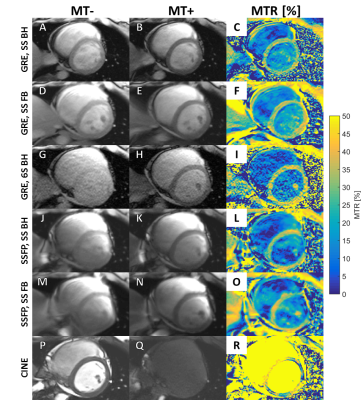
An example image set acquired with the 6 MR sequences from one subject is shown in Figure 2. The mean MTRs for each pulse sequence are shown in Figure 3A. At 3T, the SSFP sequences were confounded by minor B1 inhomogeneity artifacts in the myocardium, particularly near the inferolateral wall. The GRE BH and FB single shot mean MTRs were not significantly different from one another, although the FB sequence was graded as the best image by the cardiologist (Figure 3B). The cine-sequence demonstrated a higher MTR, but this may also be due to differences in T1 and T2 sensitivity between the 5 degree and 45 degree flip angles. This is also suggested by the high MTR in the blood pool, which should have very minor MT weighting. This technique also had the highest standard deviation of MTR values (±20.3%, as compared to the GRE BH single shot standard deviation of ±8.3%).Discussion
The free-breathing, single shot GRE MT sequence produced the highest quality MTR maps, while maintaining a low MTR standard deviation. As expected, both breath-held, single shot GRE acquisitions suffer from lower SNR than the free breathing GRE due to the smaller number of acquired images (Figure 3C). While both free breathing sequence MTRs and SNRs (GRE and SSFP) are not significantly different from one another, the SSFP sequence is more sensitive to B1-inhomogeneity effects. These artifacts produced a lower image quality score for the SSFP images (Figure 3B). The cine sequence has been previously reported for developing MTR contrast at 1.5T8 . However, at 3T, the quality of the MTR maps is reduced due to signal variations due to off-resonance effects in the myocardium.Conclusion
Single shot, free breathing MT-prepared GRE is a promising method for assessment of MTR in the heart at 3T. The technique will be further refined and evaluated in patients with heart failure.Acknowledgements
This work was supported by NIH K23 HL112910, R01 HL079110, and 2T32HL007284-41.References
1. Beltrami, C. A. et al. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 89, 151–163 (1994).
2. Weber, K. T. & Brilla, C. G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 83, 1849–1865 (1991).
3. Miller, C. A. et al. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ. Cardiovasc. Imaging 6, 373–383 (2013).
4. Perazella, M. A. Gadolinium-contrast toxicity in patients with kidney disease: nephrotoxicity and nephrogenic systemic fibrosis. Curr. Drug Saf. 3, 67–75 (2008).
5. Schlaudecker, J. D. & Bernheisel, C. R. Gadolinium-associated nephrogenic systemic fibrosis. Am. Fam. Physician 80, 711–4 (2009).
6. Scholz, T. D., Hoyt, R. F., Deleonardis, J. R., Ceckler, T. L. & Balaban, R. S. Water-Macromolecular Proton Magnetization Transfer in Infarcted Myocardium: A Method to Enhance Magnetic Resonance Image Contrast. Magn. Reson. Med. 33, 178–184 (1995).
7. Phinikaridou, A. et al. In vivo magnetization transfer and diffusion-weighted magnetic resonance imaging detects thrombus composition in a mouse model of deep vein thrombosis. Circ. Cardiovasc. Imaging 6, 433–440 (2013).
8. Weber, O. M., Speier, P., Scheffler, K. & Bieri, O. Assessment of magnetization transfer effects in myocardial tissue using balanced steady-state free precession (bSSFP) cine MRI. Magn. Reson. Med. 62, 699–705 (2009).
9. Stromp, T. A. et al. Clinical Gadolinium-Free Magnetic Resonance Imaging With Magnetization Transfer Contrast Detects Cardiac Fibrosis With High Sensitivity and Specificity Compared to Late Gadolinium Enhanced Imaging. Circulation 130, A11915–A11915 (2014).
10. Tustison, N. J., Yang, Y. & Salerno, M. Advanced Normalization Tools for Cardiac Motion Correction. in Statistical Atlases and Computational Models of the Heart - Imaging and Modelling Challenges 3–12 (Springer, Cham, 2014). doi:10.1007/978-3-319-14678-2_1
Figures