2901
Age, gender and heart rate dependency of spin echo based diffusion tensor imaging measurements in healthy hearts1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland, 2Department of Cardiology, University Hospital Zurich, Zurich, Switzerland, 3Institute of Diagnostic and Interventional Radiology, University Hospital Zurich, Zurich, Switzerland
Synopsis
Cardiac diffusion tensor imaging (cDTI) is a novel non-invasive method that allows assessing changes in myocardial microstructure in various cardiomyopathies. To identify pathologies, however, the distribution of cDTI parameters and their subject specific dependencies in normal hearts need to be known. Therefore, we investigated age, gender and heart rate dependencies of quantitative parameters derived from spin-echo based cDTI in healthy subjects. Our results display the variation of cDTI parameters in normal hearts and thereby allow gauging at which level of expected pathological changes sex and age matched reference values will be needed in future clinical practice.
Introduction
Cardiac diffusion tensor imaging (cDTI) allows for the non-invasive investigation of myocardial microstructure and tissue characterization without contrast agents[1,2]. Recent studies have shown microstructural changes that occur during the progression of diseases including hypertrophic, dilated and ischemic cardiomyopathies[3-5]. At present, either spin-echo (SE) or stimulated-echo acquisition mode (STEAM) sequences are used for cDTI. While the anthropometrics of healthy volunteers for cDTI using STEAM sequences were already investigated[6,7], these results cannot be transferred to SE based cDTI because quantitative measures of DTI including mean diffusivity (MD) and fractional anisotropy (FA) are significantly different between SE and STEAM[8]. Therefore, the objective of the present work was to investigate age, gender and heart rate dependencies of quantitative cDTI parameters derived from a SE based sequence in healthy subjects.Methods
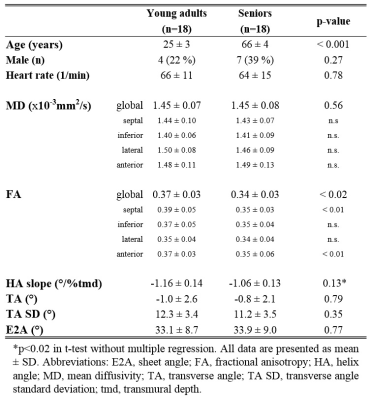
For this study, 36 volunteers (11 male, 31%) without known cardiovascular disease were recruited. The study population consisted of two subgroups, one representing young adults (20-35 yrs, n=18) while the other subgroup were seniors (60-75 yrs, n=18). All study subjects underwent CMR on a clinical 1.5T system (Philips Healthcare, Best, The Netherlands) equipped with a 5 channel cardiac coil and a gradient system delivering 80mT/m@100mT/m/ms. For cDTI, we used a diffusion-weighed second-order motion compensated spin-echo (SE) sequence[9] to acquire three imaging planes in short-axis orientation in mid systole. Prospective cardiac triggering and respiratory navigator based slice tracking[10] allowed for data acquisition during free-breathing with a spatial resolution of 2.5x2.5x8 mm3 and a FOV of 230x98 mm2. Diffusion weighting was encoded with a b-value of 450 s/mm2 along 9 and a b-value of 100 s/ms2 along 3 orthogonal directions[8]. To reduce residual motion in the data series, groupwise non-rigid image registration exploiting total variation displacement regularization and a PCA-based image similarity metric was used[11]. On the aligned data, MD, FA, helix, transverse and absolute E2A sheet angle (HA, TA, SA) were computed[12]. In addition, the transmural gradient of the HA was calculated (HA slope). All parameters were evaluated in 4 sectors of the LV at the mid-ventricular level. Differences in the baseline characteristics between both groups were tested using unpaired two-tailed t-tests. The influence of age, sex and heart rate on the diffusion parameters was evaluated using a multiple regression analysis.Results
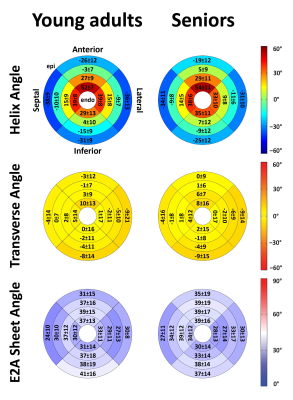
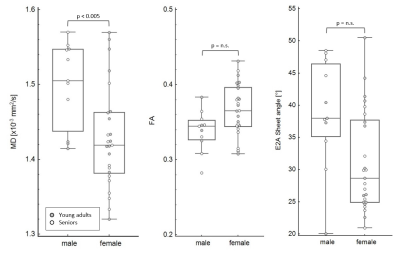
Data acquisition and analysis was successful in all study subjects. Global MD agreed closely in both age groups and also the regional distribution of the segmental MD was found to be similar in young adults and seniors (Table 1). In contrast, global FA was the only parameter that exhibited an age-dependence that was driven by significantly lower values in the septal and anterior segments of seniors. For TA, TA standard deviation (SD) and the E2A sheet angle, no differences between the groups were found. Figure 1 summarizes HA, TA and E2A for both populations. Using a t-test, the transmural slope of the HA was significantly lower in the older subjects but this trend could not be confirmed in the multiple regression analysis. The investigation of differences between sexes revealed significantly lower MD values in women while no difference could be found for FA. All other parameter were similar in male and female subjects, except for E2A sheet angle which also showed a trend to lower values in women that did, however, not reach statistical significance in the multiple regression analyses (Figure 2). No diffusion parameter exhibited a significant dependence on heart rate.Discussion
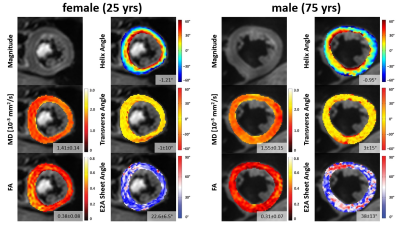
We report the first investigation of subject-specific determinants of quantitative measures of SE based cDTI. The independence on heart rate (range studied 45 – 103/min) of all cDTI parameter is an important finding for future clinical applications and an advantage compared to STEAM based sequences. STEAM based cDTI exhibits significant heart rate dependence for FA, HA slope and E2A measurements even with heart rate correction[7]. Furthermore, our results imply that the orientation of the myocardial fiberstructure, represented by HA, TA and E2A is largely conserved across age and sex in healthy hearts. In contrast, the changes found in MD and FA indicate age and sex dependent alterations of the myocardial composition within the normal fiber orientation. Figure 3 exemplarily depicts our findings by a direct comparison of the results from a young woman and an elderly man. Finally, the presented variation of cDTI parameters in normal hearts allows gauging at which level of expected pathological changes sex and age matched reference values will be needed in future clinical practice.Acknowledgements
AG and CvD contributed equally to this work.References
1. Edelman, R.R., et al., In vivo measurement of water diffusion in the human heart. Magn Reson Med, 1994. 32(3): p. 423-8.
2. Reese, T.G., et al., Imaging myocardial fiber architecture in vivo with magnetic resonance. Magn Reson Med, 1995. 34(6): p. 786-91.
3. von Deuster, C., et al., Studying Dynamic Myofiber Aggregate Reorientation in Dilated Cardiomyopathy Using In Vivo Magnetic Resonance Diffusion Tensor Imaging. Circ Cardiovasc Imaging, 2016. 9(10).
4. Sosnovik, D.E., et al., Microstructural impact of ischemia and bone marrow-derived cell therapy revealed with diffusion tensor magnetic resonance imaging tractography of the heart in vivo. Circulation, 2014. 129(17): p. 1731-41.
5. Nielles-Vallespin, S., et al., Assessment of Myocardial Microstructural Dynamics by In Vivo Diffusion Tensor Cardiac Magnetic Resonance. J Am Coll Cardiol, 2017. 69(6): p. 661-676.
6. McGill, L.A., et al., Heterogeneity of Fractional Anisotropy and Mean Diffusivity Measurements by In Vivo Diffusion Tensor Imaging in Normal Human Hearts. PLoS One, 2015. 10(7): p. e0132360.
7. McGill, L.A., et al., Relationship between cardiac diffusion tensor imaging parameters and anthropometrics in healthy volunteers. J Cardiovasc Magn Reson, 2016. 18: p. 2.
8. von Deuster, C., et al., Spin echo versus stimulated echo diffusion tensor imaging of the in vivo human heart. Magn Reson Med. 2016 Sep;76(3):862-72
9. Stoeck, C.T., et al., Second-order motion-compensated spin echo diffusion tensor imaging of the human heart. Magn Reson Med. 2016 Apr;75(4):1669-76
10. Moulin, K., et al., In vivo free-breathing DTI and IVIM of the whole human heart using a real-time slice-followed SE-EPI navigator-based sequence: A reproducibility study in healthy volunteers. Magn Reson Med, 2016. 76(1): p. 70-82.
11. Vishnevskiy, V., et al., Isotropic Total Variation Regularization of Displacements in Parametric Image Registration. IEEE Trans Med Imaging, 2016.
12. Ferreira, P.F., et al., In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson, 2014. 16: p. 87.
Figures