2899
Isotropic 3D Late Gadolinium Enhancement Imaging using 3D Patch-Based Super-Resolution1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department of Computer Science, Technical University of Munich, Munich, Germany, 3IADI, INSERM U947 and Universite de Lorraine, Nancy, France, 4Department of cardiology, University Hospital of Brabois, Nancy, France, 5CIC-IT 1433, INSERM, Nancy, France, 6Pole Imagerie, CHRU de Nancy, Nancy, France, 7Department of Cardiac Surgery, CHU Strasbourg, Strasbourg, France
Synopsis
Cardiac late gadolinium enhancement (LGE) imaging has become a reference clinical tool for assessing myocardial scar and viability. Despite superior signal-to-noise-ratio of 3D LGE techniques, current 3D breath-hold acquisitions are still limited by scan time and low-resolution, especially in the through-plane direction. Consequently, most clinical protocols include three anisotropic LGE acquisitions in different views to better visualize myocardial fibrosis in different orientations. Nevertheless, assessing myocardial viability in different views remains tedious and time-consuming. In this study, we sought to achieve isotropic 3D LGE by combining low-resolution anisotropic acquisitions using a 3D patch-based super-resolution reconstruction.
PURPOSE
Cardiac late gadolinium enhancement (LGE) imaging has become a reference clinical tool for assessing myocardial scar and viability, providing excellent prognostic risk stratification of patients after myocardial infarction (MI). Despite superior signal-to-noise-ratio (SNR) of three-dimensional (3D) LGE techniques, current 3D breath-hold acquisitions are still limited by scan time and low-resolution, especially in the through-plane direction, leading to poor image quality in that dimension. Consequently, most clinical protocols include three anisotropic LGE acquisitions in different views to better visualize myocardial scar. Nevertheless, assessing myocardial viability in different views remains tedious, time-consuming and is prone to inaccuracies due to partial volume effect (PVE). In this study, we sought to achieve isotropic 3D LGE by combining low-resolution anisotropic acquisitions using a 3D patch-based super-resolution (SR) reconstruction. The technique is demonstrated on patients with MI or focal fibrosis, where high isotropic resolutions are often required to robustly assess the location and extent of myocardial fibrosis.METHODS
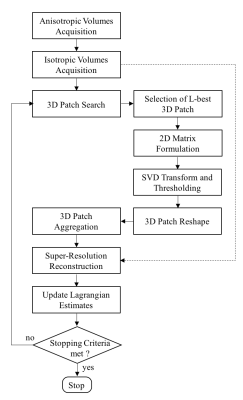
The proposed isotropic 3D reconstruction scheme integrates anatomical structure information from 3D patch neighbourhoods through sparse representation, exploiting the redundancy of non-local 3D patches in the acquired data itself. The optimization problem iterates between a SR reconstruction1, which creates a high-resolution isotropic volume from anisotropic acquisitions, and a low-complexity 3D patch-based reconstruction which enables high-SNR reconstruction (Fig.1). The two sub-problems are solved iteratively into an effective Augmented Lagrangian (AL) scheme:
Super-Resolution: Given $$$Z$$$ anisotropic measurements ($$$Z=3$$$ in this study), representing the same object in nearly orthogonal directions, the isotropic volume $$$x$$$ is found by solving the following regularized least squares optimization problem:
$$\underset{x}{\mathrm{argmin}} \,\frac{1}{2}\Vert SBTx-\rho\Vert_2^2+\frac{\mu}{2}\Vert x-\omega-b\Vert_2^2$$
where $$$T$$$ is a rigid image transformation that takes the SR image from the desired reconstructed orientation to the orientation of the ith acquisition, $$$SB$$$ is a slice selection operator including a blurring operator $$$B$$$ and a downsampling operator $$$S$$$. This information is extracted from the images’ DICOM file headers. The regularization parameter $$$\mu$$$ imposes the degree of closeness to the acquired data, $$$b$$$ denotes the AL parameter and $$$\omega$$$ the high-SNR isotropic volume obtained with the following 3D-patch reconstruction.
3D-Patch: A 3D block-matching2,3 algorithm is used to exploit redundancies in the reconstructed volume. 3D blocks are extracted and compared to a reference block using the l2-norm distance. The L-most similar blocks to the reference are selected, vectorised and concatenated into a sparse 2D matrix. This 2D matrix contains a high degree of non-local similarity and redundancy and therefore exhibits a low-rank structure. Sparsity of the matrix is enforced using singular values decomposition and by hard thresholding the singular values below a specific threshold. The denoised 3D blocks are then placed back to their original positions by averaging.
Imaging: Thirteen patients (13 men, 56±13 years) with a remote (>6 months) MI underwent CMR using a 1.5T GE Signa system with an 8-channel cardiac coil. Three additional patients with Duchenne muscular dystrophy were recruited and underwent conventional LGE imaging. Three breath-held 3D LGE were performed 15 minutes after Gadolinium injection with the following acquisition parameters: TR/TE=3.46/1.28ms, TI adjusted to cancel healthy myocardial signal, matrix=256x256x20, AT=~15s x 3 breath-holds, with 1.25mm in-plane resolution and 4.5/8mm slice thickness. The proposed technique was used to reconstruct a single 3D isotropic volume of resolution 1.25x1.25x1.25mm3 with the following parameters: block size=15x15x15, L=100, $$$\mu=1$$$, and AL-iterations=6. The reconstructed volumes were compared to conventional SR reconstructions (Beltrami1, Tikhonov1) and were evaluated for quality by two experienced cardiologists.
RESULTS
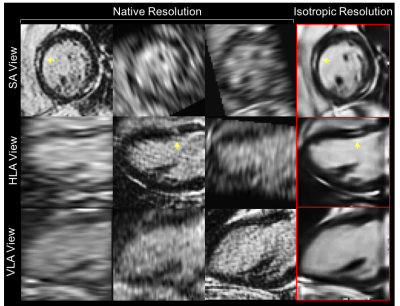
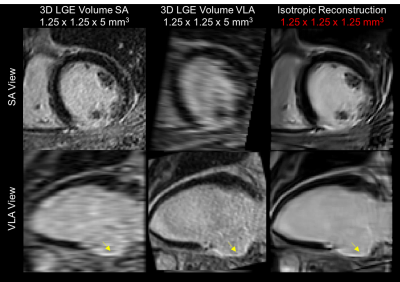
Reconstruction of a 20-year-old Duchenne patient with focal fibrosis is shown in Fig.2. The reconstructed isotropic volume was visually better with a higher overall SNR and a satisfactory CNR. The isotropic 3D reconstruction attenuates PVE and provides a fibrosis area which is consistent between the different views. The isotropic reconstruction from a 78-year-old patient with an inferior MI scar is shown in Fig.3. The proposed reconstruction technique yields significantly better overall SNR, reducing the noise in flat areas while preserving thin anatomical structures such as fat, papillary muscles and myocardial scar. The proposed reconstruction was ranked as the best image in 16/16 patients for the first expert (15 patients for the second expert) with an excellent inter-observer reproducibility ($$$\kappa=0.875$$$).DISCUSSION
We demonstrate the feasibility of integrating non-local structure information into a SR framework. The proposed technique allows unrestricted reformatting in any desired plane without loss of resolution. Preliminary results in clinical cardiac LGE show that the reconstructed isotropic 3D volume provides improved information to the cardiologist, at no cost for assessing the location and transmural extent of myocardial fibrosis. Ultimately, this technique may be helpful for in progress ventricular arrhythmia risk assessment methods.4Acknowledgements
The authors received support from the European Commission through Grant Number 605162.References
1. Odille F, et al. Motion-corrected, super-resolution reconstruction for high-resolution 3D cardiac cine MRI, MICCAI, 2015;9351:259-268.
2. Akçakaya M, et al. Low-dimensional-structure self-learning and thresholding (LOST): regularization beyond compressed sensing for MRI reconstruction, Mang. Reson. Med, 2011;66(3):756:767.
3. Dabov K, et al. Image denoising by sparse 3D transform-domain collaborative filtering, IEEE TIP, 2006;16(8):2080-2095.
4. Cochet H, et al. Integration of merged delayed-enhanced magnetic resonance imaging and multidetector computed tomography for the guidance of ventricular tachycardia ablation: a pilot study, J Cardiovasc Electrophysiol, 2013;24(4):419-426.
Figures