2898
Dynamic Nitroxide-Enhanced MRI Detects Oxidative Stress in Myocardial Infarction1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Radiology, University of Virginia, Charlottesville, VA, United States
Synopsis
Oxidative stress plays an important role in the pathogenesis of myocardial repair and remodeling after myocardial infarction (MI). Nitroxide free radicals have been used as redox-sensitive MRI contrasts agents in preclinical studies to assess tumor redox status. We tested the hypothesis that dynamic nitroxide-enhanced MRI can detect oxidative stress in MI. Imaging was performed in healthy control mice and in mice one day post-MI. The ratio of the MRI signal decay between the infarcted anterolateral wall and the noninfarcted septum was significantly higher in mice after MI, indicating that nitroxide-enhanced MRI can detect increased oxidative stress in infarcted myocardium.
Introduction
Oxidative stress, defined as the excess production of reactive oxygen species (ROS) relative to antioxidant reserves, plays an important role in the pathogenesis of myocardial repair and remodeling after myocardial infarction (MI) and in other cardiovascular diseases.1-3 Following MI, NADPH oxidase dependent oxidative stress develops in the infarcted myocardium with neutrophils and macrophages as the primary cells expressing the enzyme.3,4 Techniques to measure oxidative stress through serum and circulating biomarkers have been developed and utilized,5 however, there are no established, widely-available noninvasive methods to quantify in vivo oxidative stress localized to the heart. Nitroxide stable free radicals can serve as T1-shortening contrast agents that lose their T1-shortening property as they undergo in vivo reduction reactions. Given this property, nitroxides have been used as redox-sensitive MRI contrast agents in preclinical cancer imaging studies to assess tumor redox status.6,7 We tested the hypothesis that dynamic nitroxide-enhanced MRI can detect oxidative stress in infarcted mouse hearts. Specifically, we studied healthy control mice and mice one day after reperfused MI. We hypothesized that the decay rate of the nitroxide-enhanced MRI signal would be elevated in infarcted compared to noninfarcted myocardium, indicative of oxidative stress.Methods
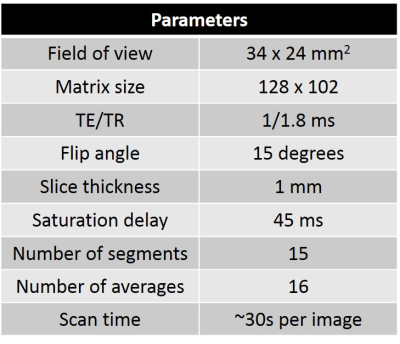
The nitroxide contrast agent 3-Carbamoyl-PROXYL (3CP) (Sigma–Aldrich, St. Louis, MO) was chosen because it is water soluble, commercially available, well tolerated by mice, has a relaxation rate R1 that increases linearly with concentration in the range of 0.5 to 35 mM, and it provides myocardial enhancement. The relaxivity of 3CP in saline solution at 7T was previously measured to be 0.139 mM-1sec-1. MI was induced in mice by a 40 minute left coronary artery (LCA) occlusion followed by reperfusion. Wild type male C57Bl/6 mice one day post-MI (n = 6) and healthy control mice (n = 10) underwent MRI studies using a 7T system (Clinscan, Bruker). The electrocardiogram (ECG), body temperature, and respiration were monitored during imaging (SA Instruments, Stony Brook, NY). During MRI, mice were anesthetized with 1.25% isoflurane and maintained at 36 ± 0.5°C using circulating warm water. After localizer imaging, DENSE MRI8 and proton-density weighted MRI were performed, and serial T1-weighted MRI was performed in a mid-ventricular short-axis slice before and consecutively after 3CP injection for 10 minutes. 3CP was administered through an indwelling tail vein catheter at 2 mmol/kg body weight over 3 to 4 seconds. The concentration of 3CP in the bolus solution was 50 mg/mL. ECG-gated saturation-recovery rapid gradient echo imaging was used for T1-weighted MRI (Table 1). DENSE circumferential strain maps were used to identify infarct and remote noninfarcted regions of interest (ROIs) in the MI mice, which are consistently in the anterolateral and septal segments, respectively, for this LCA occlusion model. In control mice, anterolateral and septal ROIs were manually delineated. These ROIs were applied to the nitroxide-enhanced images. ROI signal intensities from nitroxide-enhanced images were normalized by their proton-density signal intensities and were converted to 3CP concentrations using the methods described by Cernicanu and Axel.9 The ROI 3CP concentration vs. time curves were analyzed, and the decay rate from 3-10 minutes after 3CP injection was calculated by least-squares fitting of the following equation: ln(3CP concentration) = constant – decay_rate * time. The ratios of infarct to remote ROI signal decay rate in infarcted mice and anterolateral to septal ROI signal decay rate in control mice were then compared.Results
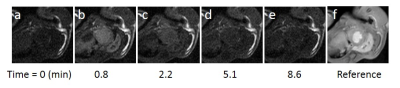
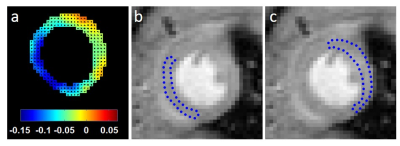
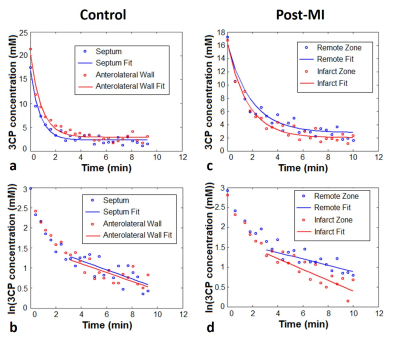
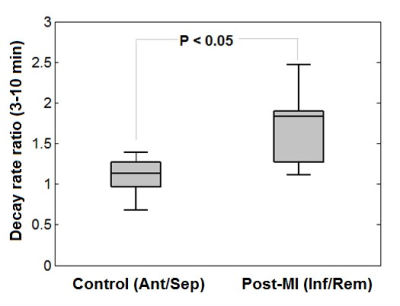
Figure 1 shows example MR images before and after injection of 3CP, showing initial 3CP signal enhancement and then decay over time. Figure 2 shows an example DENSE circumferential strain map used to identify infarcted and remote ROIs in the anterolateral wall and septum, respectively. Figure 3 shows example [3CP] vs. time and ln[3CP] vs time curves obtained from mice along with 3-10 minute linear fits of the two ROIs for a healthy mouse (a,b) and a post-MI mouse (c,d). Figure 4 summarizes the data from all mice showing that the 3-10 minute decay rate ratio is significantly greater in MI mice compared to controls (1.73 ± 0.20 MI vs 1.10 ± 0.06 controls; p<0.05).Conclusion and Discussion
Using nitroxide-enhanced MRI, we detected an elevated decay rate ratio in MI mice compared to control mice, indicating that nitroxide-enhanced MRI can detect increased oxidative stress in infarcted myocardium. The enhanced decay rate ratio cannot be explained by decreased myocardial perfusion in the infarcted region, as this would manifest as a reduced decay rate. These methods can be applied in preclinical studies that seek to investigate the mechanistic role or therapeutic manipulation of oxidative stress during scar healing and post-MI left-ventricular remodeling.Acknowledgements
Funding: NIH R01 EB001763 and NIH training grant T32 HL007284References
1. Tsutsui, H., S. Kinugawa, and S. Matsushima, Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol, 2011. 301(6): p. H2181-90.
2. Dhalla, N.S., R.M. Temsah, and T. Netticadan, Role of oxidative stress in cardiovascular diseases. J Hypertens, 2000. 18(6): p. 655-73.
3. Yao, S., Myocardial repair/remodeling following infarction: roles of local factors. Cardiovascular Research, 2008. 81(3): p. 482-490.
4. Mohazzab-H KM, Kaminski PM, Wolin MS. Lactate and PO2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation, 1997. 15: p. 614–620.
5. Ho, E., et al., Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol, 2013. 1: p. 483-91.
6. Hyodo, F., et al., Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res, 2006. 66(20): p. 9921-8.
7. Matsumoto, K., et al., High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin Cancer Res, 2006. 12(8): p. 2455-62.
8. Aletras, Anthony H., et al. DENSE: Displacement Encoding with Stimulated Echoes in cardiac functional MRI. Journal of Magnetic Resonance, 1999. 137(1): p. 247-252.
9. Cernicanu, A. and L. Axel, Theory-based signal calibration with single-point T1 measurements for first-pass quantitative perfusion MRI studies. Acad Radiol, 2006. 13(6): p. 686-93.
Figures