2881
Observation of the kinetics of antioxidant action in blood serum as measured by NMR relaxation1Food Technology, University of Agriculture in Krakow, Krakow, Poland, 2Pedagogical University, Krakow, Poland, 3Institute of Physics, Jagiellonian University, Krakow, Poland
Synopsis
The exposure of blood serum to reactive oxygen species creates free radicals and damages the structure of biomolecules. It causes proton NMR relaxation times to change over time. In aqueous protein solutions, T1 and T2 decay curves were fitted by single exponential components. Following addition of hydrogen peroxide to blood serum which contained endogenous antioxidants, relaxation times initially decreased then increased towards initial values. The character of these curves and their fitting parameters depend on kinetics of oxidation processes. We hope that MR imaging may help to investigate these important processes also in vivo.
Introduction
Oxidation processes occur in a wide variety of human chronic diseases. There is therefore a need for monitoring and regulation of these processes by measuring levels and activity of endogenous antioxidants in blood. Recently, we measured the time behavior of relaxation times T1 and T2, after initiation of an oxidation process by addition of 3% hydrogen peroxide to blood serum. In aqueous protein solution (like egg white or bovine serum albumin) measured time courses were well fitted by exponential decays in which the parameters depended on the type and concentration of proteins [1]. Addition of an antioxidant (e.g. ascorbic acid) to protein solution caused the measured relaxation times to increase towards initial values. Similar non-exponential time courses were observed in native blood serums which contained endogenous antioxidants [2]. In this study we model the time course of relaxation time behavior during the action of antioxidants. The parameters obtained are sensitive to the activity and the concentrations of antioxidants. Therefore NMR relaxation may be a good tool for investigation of oxidation processes in biological materials.Methods
Fresh human and dog blood serum samples, treated with citric acid, were obtained from common diagnostic stations as an excess material after routine investigation. NMR relaxation times were measured using Minispec Bruker spectrometer operating at 1.5 T (60 MHz) at room temperature (+24oC) using IR (T1) and CPMG sequence (T2) methods. Data were analyzed using standard Bruker software and measurement errors were about 2%.Results
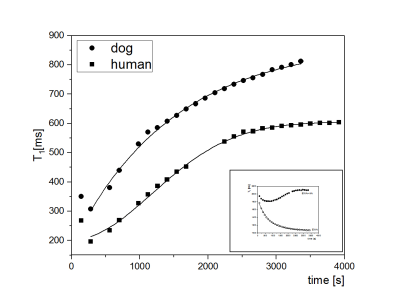
Fig.1 shows the time course of T1 after addition of 3% aqueous solution of H2O2 in proportion 1:10 (by weight) to native human and dog blood serum. Initial values of T1 were 1770±20 ms (human) and 1636±40 ms(dog). After addition of hydrogen peroxide the value of T1 dropped significantly to 196±10 ms and 308±20 ms respectively. All time courses in serum showed the same characteristics: a very short decay at the beginning followed by an increase until a plateau was reached. This behavior was similar to time courses of aqueous protein solution with addition of ascorbic acid as an antioxidant [1]. Similar times courses were obtained also in T2 measurements from the same sample. Although the T2 was much shorter, the measurements of T1 proved to be more useful for observation of kinetics of oxidation processes because of the independence of T1 on H2O2 concentration up to high concentrations [2]. The time course of the human serum after addition of H2O2 and after a rapid decay of T1 can be fitted by the Gaussian function according to the formula:
$$T_{1h}(t)=T_{h0}-T_{h}e^{(-\frac{t}{t_{h}})^2} (1) $$
and the parameters obtained are:
Th0 = 604,9±0,5 ms (equilibrium value of T1 relaxation time),
Th = 402,4±0,7 ms (the gain in value of T1 relaxation time),
th = 1671±4 s (the kinetics time constant of antioxidant action).
The time course for dog serum sample after addition of H2O2 and after rapid decay at the beginning can be fitted by an exponential function according to formula:
$$T_{1d}(t)=T_{d0}-T_{d}e^{(-\frac{t}{t_{d}})} (2)$$
where the parameters are: Td0 = 899,3±9,7 ms (equilibrium value of T1 relaxation time),
Td = 787,2±13,1 ms (the gain in value of T1 relaxation time),
td = 1057±50 s (the kinetics time constant of antioxidant action).
The antioxidant action is significantly faster for dog serum than for human serum (th>td), that may be related to types and amounts of serum proteins responsible for this activity. The mechanism of action has to be different too, as it is described by other models of oxidation processes [3].
Conclusions
These results show that the measurements of time courses of T1 after addition of H2O2 for initiation oxidative processes can be used to observe the kinetics of antioxidant action. Because of non-invasiveness this NMR approach may be used in vivo. Recent research demonstrated a new technology of 3D dosimetry in radiation therapy based on the creation of free radicals, generated by ionizing radiation which influence MR relaxation times [4]. Relaxation times are very sensitive to the presence of free radicals and T1 or T2 measurements can be carried out in vivo. Therefore, we hope that, in the future, the effects of antioxidants on oxidation processes can be explored in humans in vivo.Acknowledgements
No acknowledgement found.References
1. Wierzuchowska D., Skorski L.W., Blicharska B., NMR Relaxation Measurements as a Tool for Observation of Oxidative Processes, Acta Phys. Polon. A. 2016;129(2): 226-228.
2. Skorski L.W., Solnica B., Blicharska B., Investigation of Oxidative Processes of Blood Serum using NMR Relaxation Methods, Journal of Laboratory Diagnostics, 2011;471:85-89.
3. Lecca. P., On the mathematical structure of chemical kinetic models. Proceedings of Int. Conference on Cellular and Molecular Biology, World Academy of Science, Engineering and Technology, Rome, April 2009, 2009; vol. 52: 345-368.
4. Berg A., MR imaging for 3-dimentional dosimetry in radiation tumor therapy: recent results on photon needle beams, Proceedings of 9th Krakow Workshop on Novel Applications of Imaging and Spectroscopy in Medicine, Biology and Material Science, Krakow, Poland, September 2017.
Figures