2874
SNR analysis of retrospectively gated DENSE at 7T for the measurement of brain tissue pulsatility1Radiology, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
Measurements of brain tissue pulsatility can provide information about viscoelastic tissue properties and assess microvasculature blood volume pulsations as a biomarker. VCG-triggered DENSE is capable of acquiring micrometer-level tissue displacements and volumetric strain. However, it is slow and suffers from triggering issues, especially at 7T. In this work, retrospectively-gated DENSE using a pulse oximeter was implemented at 7T. Assessment of its performance showed maintained SNR within half the scan time of triggered DENSE. The high SNR and reduced scan time simplifies its application in future studies assessing the potential of DENSE-derived brain tissue displacements as a biomarker for neurological diseases.
Introduction
Brain tissue pulsatility may prove to be a valuable biomarker for cerebral small vessel disease and other neurological disorders since these measurements can provide information about the viscoelastic tissue properties1 and the underlying microvasculature which drives this motion. Successful measurements of brain tissue pulsatility were made using a prospective vector cardiogram (VCG) triggered implementation of displacement encoding using stimulated echoes (DENSE)2,3. However, this approach suffered from long scan times and difficulties with consistent triggering with respect to the distorted VCG signal, especially at ultrahigh field (>=7T). To address these issues, a retrospective DENSE that is triggered with a pulse oximeter (POx) was implemented at 7T and used to measure the brain tissue motion in healthy volunteers.Methods
Measurement
Informed consent was obtained from 8 healthy subjects (5 males, mean age:26.8±6) to participate in this study, which was approved by the Ethical Review Board of our institution. A 4D retrospectively-gated DENSE sequence was implemented on a 7T scanner (Philips Healthcare) which was used in conjunction with a 32-channel head coil (Nova Medical) and a POx to measure the cardiac-induced whole brain tissue displacement in the right-left (RL) direction for the entire cardiac cycle. A 3D T1-weighted FFE image was also made of each subject (see Table 1 for all scan parameters). In order to assess the SNR performance of the retrospective DENSE a noise measurement was made by repeating the acquisition without gradients or RFs present.
Analysis
Displacement maps were derived from the raw phase images as previously described4. 3D SNR maps were created for each acquired cardiac phase by dividing the mean magnitude image (derived from 2 dynamics) by the modulus of the standard deviation of the complex noise5, as defined in the equation
$$SNR[p,q,r] = \frac{\textit{mean}\left \{ Mag[i,j,k] \right \}}{\sqrt{\mathit{variance} \left \{ \Re (Noise[i,j,k]) \right \} + \mathit{variance} \left \{ \Im (Noise[i,j,k]) \right \} } } $$
where $$$(i,j,k) \in w$$$, a 15x15x15 window centered around the SNR map at location $$$[p,q,r]$$$.
To create a whole brain tissue mask, the T1-weighted image was first segmented using the Computational Anatomy Toolbox (Jena University Hospital) for SPM12. The tissue mask was then applied to the DENSE and SNR images which were both registered with a rigid transformation to the T1-weighted image space using Elastix6,7. The mean SNR for all voxels contained in the tissue mask was calculated for each cardiac phase. The SNR coefficient of variation (CoV) was also calculated to assess the stability of the acquired signal over the cardiac cycle.
Results
Displacement maps were successfully obtained for all subjects. Figure 1 shows typical RL displacement and SNR maps constructed for a subject. SNR curves for all subjects are shown in Figure 2. The mean SNR for all subjects in this study was 26.1±1.5. The CoV was below 5% for all subjects (Table 2).Discussion
Although the study protocol was largely similar between triggered4 and retrospectively-gated DENSE, the SNR performance of retrospectively-gated DENSE appears to be better than that of triggered DENSE. (The mean SNR for 8 subjects previously measured using triggered DENSE4 was 21.2±6.3). Unlike the triggered DENSE, the retrospectively-gated DENSE did not utilize a saturation pulse to remove remaining signal at the end of the last cardiac phase, which may account for the higher SNR observed in this study. Volumetric strain is derivable with further measurements of displacements in the AP and FH directions. This metric holds potential as means for quantifying cardiac-induced blood volume changes in the microvasculature as it may yield a useful biomarker for neurological disease. For this purpose, the consistent triggering of the POx reduces the possibility of de-synchronicity between acquired RL/AP/FH datasets. Additionally, the improved speed without SNR penalty in comparison with triggered DENSE further reduces possible phase corruption due to gross subject motion. The SNR curves of the subjects noticeably dips slightly before increasing towards the end of the cardiac cycle, which is a consequence of the retrospectively gated algorithm interpolating the cardiac phases. It may also reflect the fact that there is less data for this point in the cardiac cycle, given the interruption of scanning after a trigger to impose the next DENSE encoding. The low CoV suggests good signal stability of the cardiac cycle despite the T1 dependence of the variable flip angles used8, compounded with the spatial variation of the applied flip angle due to B1 inhomogeneity.Conclusion
We successfully implemented a retrospectively-gated DENSE at 7T and demonstrated that it is a more SNR efficient method for measuring brain pulsatility than triggered DENSE. The halved scan time without SNR penalty will simplify its application in future studies assessing the potential of brain tissue pulsatility measured with DENSE as a biomarker for neurological diseases.Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n°337333.References
1. Weaver JB, Pattison AJ, McGarry MD, Perreard IM, Swienckowski JG, Eskey CJ, Lollis SS, Paulsen KD. Brain mechanical property measurement using MRE with intrinsic activation. Phys. Med. Biol. 2012;57:7275–7287. doi: 10.1088/0031-9155/57/22/7275.
2. Soellinger M, Rutz AK, Kozerke S, Boesiger P. 3D cine displacement-encoded MRI of pulsatile brain motion. Magn. Reson. Med. 2009;61:153–162. doi: 10.1002/mrm.21802.
3. Adams AL, Luijten PR, Zwanenburg JJM. Measurements of cardiac related pulsatile volumetric strain in grey and white matter brain tissue with high resolution DENSE at 7T. In: Proceedings of the 25th Annual Meeting of ISMRM. Honolulu, USA; 2017.
4. Adams AL, Luijten PR, Zwanenburg JJM. An SNR analysis of DENSE at 7T vs 3T for the measurement of whole brain tissue pulsatility. In: Proceedings of the 25th Annual Meeting of ISMRM. Honolulu, USA; 2017.
5. Henkelman RM. Measurement of signal intensities in the presence of noise in MR images. Med. Phys. 1985;12:232–233. doi: 10.1118/1.595711.
6. Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. Elastix: A toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging 2010;29:196–205. doi: 10.1109/TMI.2009.2035616.
7. Shamonin DP, Bron EE, Lelieveldt BPF, Smits M, Klein S, Staring M. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front. Neuroinform. 2013;7:50:1-15. doi: 10.3389/fninf.2013.00050.
8. Stuber M, Spiegel MA, Fischer SE, Scheidegger MB, Danias PG, Pedersen EM, Boesiger P. Single breath-hold slice-following CSPAMM myocardial tagging. Magn. Reson. Mater. Physics, Biol. Med. 1999;9:85–91. doi: 10.1016/S1352-8661(99)00049-6.
Figures
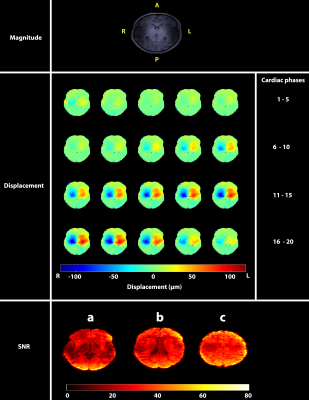
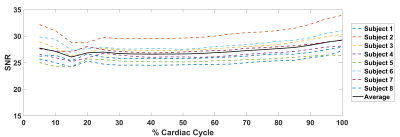
Table 1. Imaging parameters used in the study.
- The flip angles were varied over the cardiac cycle to create a constant signal response8, assuming a T1 of 1100 ms and taking a max. flip angle of 30o.
- The retrospectively-gated DENSE acquisition was performed using a pulse oximeter as the trigger device, which was attached to the left index finger.
- The reported scan duration is for a heart rate of 60 beats/min.