2863
Data processing methods for the extraction of novel FFC-MRI biomarkers1University of Aberdeen, Aberdeen, United Kingdom
Synopsis
Fast Field-Cycling (FFC) MRI generates images with T1-dispersion contrast that provide new insights for medical applications. No model of such dispersion data exists for biological tissues therefore a phenomenological approach is chosen here that minimises data loss while isolating meaningful information by curve fitting. This approach provided promising biomarkers in several pilot studies spanning a range of applications: osteoarthritis, liver fibrosis, breast cancer, glioma and fatty tissues. This shows that a dispersion-based approach of FFC-MRI data is an interesting and novel approach for the discovery of novel biomarkers.
Introduction
Fast Field-Cycling (FFC) MRI allows accessing T1 dispersion information over 3 to 5 decades of magnetic field strength 1. This provides exciting opportunities to find novel and relevant biomarkers of diseases. However the traditional approach of T1 dispersion analysis often relies on a field-by-field comparison approach, which misses important information, or on the application of a model, which is not currently possible for in-vivo studies. Here we present a phenomenological approach to the analysis of T1 dispersion data and some of the results that it has provided to date.Methods
T1 dispersion curves were extracted from FFC-NMR or FFC-MRI experiments using in-house software, and then analysed by curve fitting using a least-square minimisation approach. The model used for the fit is derived from models described in the literature 2–6 with modifications to avoid loss of information. These modifications consisted in adding models together, selecting certain models over specific regions of the spectrum or adding exponents to the field term in the equation, and were chosen on the basis of greatest simplicity while preserving non-biased and normal-distributed residuals. Once the optimal model was found the parameters obtained for each dispersion data were compared with the corresponding clinical findings. This approach has been used on T1-dispersion data from FFC-NMR of resected tissues and from FFC-MRI images of human volunteers and patients. These were obtained from pilot studies focusing on osteoarthritis, breast cancer, musculoskeletal sarcoma, fatty tissues, brain glioma and liver fibrosis.Results
Most tissues analysed so far were correctly fitted using piecewise power laws, stretched Lorentzian and quadrupolar peaks. Power laws appeared mostly in fatty tissues, muscle, tumours and brain while stretched Lorentzian profiles were observed in healthy liver. Quadrupolar peaks appeared everywhere with various amplitudes except in fatty tissues and in some glioma where they were absent. Most tissues showing power law-like dispersions had normal-distributed residuals after up to three segments. The number of segments used to fit these curves was increased until there was no significant difference between the segment added and the previous one.Discussion
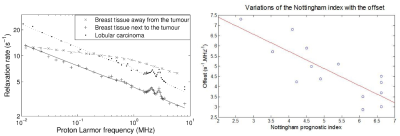
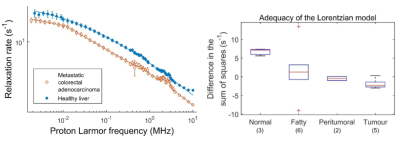
This approach has provided several potential biomarkers: the average T1 value, which correlated strongly with patient prognosis in breast cancer (Figure 1), the number of segments in the power laws, which differed for grain glioma, the switch from stretched Lorentzian to power law, which was observed in liver fibrosis (Figure 2), the amplitude of the quadrupolar peaks, which was associated with cartilage disease in osteoarthritis and oedema, and the intensity of the dispersion, which varied with tumour types. Preliminary work using proteomics shows possible relationships with biological pathways such as fibrinolysis, necrosis or metabolism in the case of glioma.Conclusion
The analysis of T1 dispersion, as opposed to T1 values at specific fields, provides potential clinical biomarkers in a variety of pathologies that seem to be strongly linked to the molecular activity in the tissues and to provide information on tissue remodelling. The examples shown here illustrate the high potential for such an approach for medical application and uncover some of the possible biomarkers found in the context studied; many more applications are to be investigated, especially since FFC-MRI now allows measuring these in-vivo non-invasively.
Acknowledgements
This work was supported by EPSRC [grant numbers EP/E036775/1, EP/K020293/1] and received funding from the European Union’s Horizon 2020 research and innovation programme [grant agreement No 668119, project “IDentIFY”].
References
1. Lurie, D. J. et al. Fast field-cycling magnetic resonance imaging. Comptes Rendus Phys. 11, 136–148 (2010).
2. Kimmich, R. & Anoardo, E. Field-cycling NMR relaxometry. Prog. Nucl. Magn. Reson. Spectrosc. 44, 257–320 (2004).
3. Korb, J.-P. & Bryant, R. G. Magnetic field dependence of proton spin-lattice relaxation of confined proteins. Comptes Rendus Phys. 5, 349–357 (2004).
4. Koenig, S. H. & Brown, R. D. A molecular theory of relaxation and magnetization transfer: application to cross-linked BSA, a model for tissue. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med. 30, 685–695 (1993).
5. Kruk, D., Herrmann, A. & Rössler, E. A. Field-cycling NMR relaxometry of viscous liquids and polymers. Prog. Nucl. Magn. Reson. Spectrosc. 63, 33–64 (2012).
6. Fries, P. H. & Belorizky, E. Simple expressions of the nuclear relaxation rate enhancement due to quadrupole nuclei in slowly tumbling molecules. J. Chem. Phys. 143, 044202 (2015).
Figures