2839
Infant brain extraction in T2 weighted MR images using k-means clustering and spatial information1Gachon Advanced Institute for Health Science and Technology (GAIST), Gachon University, Incheon, Republic of Korea, 2Neuroscience Research Institute, Incheon, Republic of Korea, 3College of Health Science, Gachon University, Incheon, Republic of Korea
Synopsis
Brain extraction is an essential pre-processing step for brain image analysis. In this work, a new brain extraction technique for T2 weighted image of an infant brain with pathological characteristics is proposed to reduce the error of conventional techniques caused by variations in contrast and brain size of infant brain from that of the adult brain. We used k-means clustering, spatial information, and morphological approaches to improve brain extraction technique. Quantitative analysis was conducted using the dice ratio compared with the results of manual segmentation.
Introduction
Brain extraction1,2 is an essential pre-processing step for a wide variety of brain image analysis methods, such as estimation of brain volume, observation of morphological changes, segmentation of sub-volumes, etc. Most brain extraction algorithms are optimized for adult T1 weighted images. When only T2 weighted image is available for image analysis, the number of applicable brain extraction algorithms may be much less. Moreover, if it is the case for infant brains, especially with pathological characteristics, applying the conventional brain extraction algorithms may not provide accurate results because an infant’s brain is still under development. Thus, this abstract introduces an optimized brain extraction algorithm for infant T2 weighted image.Methods
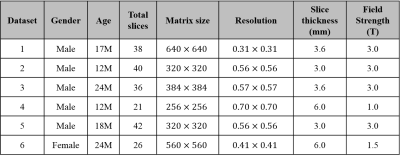
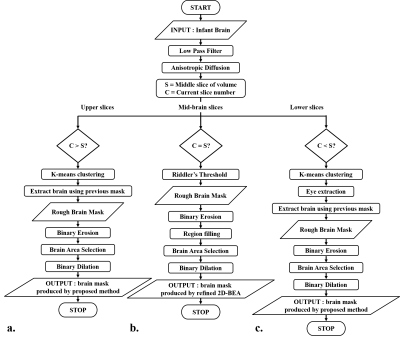
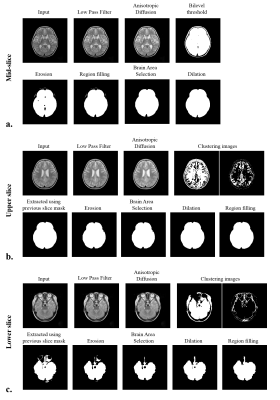
We used infant (age: 2~24 months) T2 weighted images (Table 1). Datasets contain images from normal infants and infants with pathological characteristics expressed as hypo or hyper-intensities in MR image. The flowchart of the proposed method is illustrated in Fig. 1. First, the mid-slice of the brain volume is selected with following procedure; 1) finding a threshold using Riddler’s method as in 2D-BEA3 and generating a rough mask, 2) getting the mid-slice mask using morphological approaches (erosion, region filling, largest connected components (LCC), dilation). With respect to the position of the mid-slice, the brain images are categorized into upper and lower slices so that the lower slices containing the eyeballs can be processed differently. The upper slices are then processed from the center towards upper slices using following procedures; 1) applying the low pass filter and the anisotropic diffusion, 2) classifying the 7-classes binary images using the K-means clustering, 3) extracting the brain using the mask of the previous slice, 4) performing erosion, 5) getting the largest region using LCC, 6) applying dilation to recover erosion brain mask. Lower slices are processed from the center towards lower slices with the following procedures; steps 1) and 2) of the upper slices processing. 3) extracting eye from the clustered image, 4) removing non-brain region using spatial information of the previous slice, performing steps, 4), 5), and 6) of the upper slices processing. For step 5 of the lower slices processing, part of the brain regions that are not included in the largest component in LCC can be removed. To solve this problem, overlap test in 3D-BEA was applied with optimized parameters. Finally, we conducted region filling to eliminate remaining holes within brain mask (Fig. 2).Results
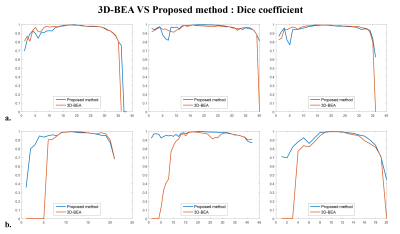
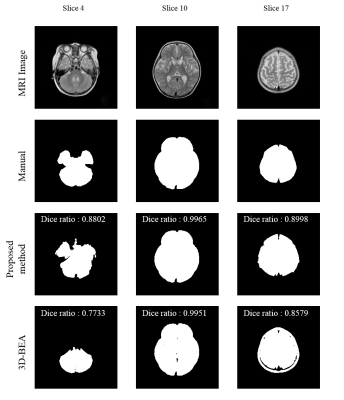
We evaluated T2 weighted images from six infant subjects (age: 12~24 months, mean±std = 17.83±4.91), which include 3 normal images (Datasets No. 1~3 in Table 1) and 3 images with hypo or hyper-intensity regions (Datasets No. 4~6 in Table 1). The dice ratio is used for measuring the accuracy of the extracted brain by comparing manual segmentation with the proposed method and 3D-BEA3. In normal images, the averaged dice ratio of 3D-BEA was 0.9139 (std, 0.19) and that of the proposed method was 0.9259 (std, 0.11). In images with hypo or hyper-intensities, the averaged dice ratio of 3D-BEA was 0.7454 (std, 0.36) and that of the proposed method was 0.9158 (std, 0.10). In total images, averaged dice ratio of 3D-BEA was 0.8297 (std, 0.28) and that of the proposed method was 0.9208 (std, 0.11). In normal images, the proposed method and 3D-BEA did not show significant differences. However, the proposed method showed significantly improved performance than 3D-BEA in the images with abnormal intensities, and the proposed method showed higher dice ratio and lower variance in total dataset. As shown in Figs. 3a and 3b, the proposed method showed significant improvement of dice ratio in lower slices than that of 3D-BEA.Discussion
The proposed method reduced the errors of brain extraction algorithm in T2 weighted images of infant with abnormal intensities. We used k-means clustering algorithm to include the brain region with abnormal intensities in the brain mask. However, non-brain regions can be falsely included in the brain mask by the errors of k-mean clustering algorithm. Thus, we used the segmentation result of previous slice to remove non-brain regions from the brain mask.Conclusion
Brain extraction is an essential pre-processing step for a wide variety of brain image analysis methods. In this work, we proposed a new brain extraction algorithm for T2 weighted images of infant brain, which is based on the k-means clustering. The quantitative analysis using the dice ratio and the quantitative comparison of segmenation results showed that the proposed method showed improved performance compared with that of the 3D-BEA especially in the images with abnormal intensities (Fig. 4).Acknowledgements
This research was partly supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014M3C7033999) and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry for Health and Welfare, Korea (HI14C1135).References
1. S.M. Smith. Fast robust automated brain extraction. Human Brain Mapping, 17(3):143-155, November 2002.
2. David W. Shattuck, Stephanie R. Sandor-Leahy, Kirt A. Schaper, David A. Rottenburg, Richard M. Leahy. "Magnetic Resonance Image Tissue Classification Using a Partial Volume Model." NeuroImage 2001; 13(5):856-876
3. K. Somasundaram, T. Kalaiselvi Fully automatic brain extraction algorithm for axial T2-weighted magnetic resonance images. Comput. Biol. Med., 40 (2010), pp. 811-822, 10.1016/j.compbiomed.2010.08.004
Figures