2837
A software package designed to integrate advanced fMRI methods for presurgical mapping and clinical studies (IClinfMRI)1Department of Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan, 3Department of Diagnostic Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 4Graduate Institute of Humanities in Medicine, Taipei Medical University, Taipei, Taiwan, 5Section of Neuropsychology, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 6Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Task-evoked and resting-state (rs) fMRI techniques have been applied to clinical management of neurological diseases, exemplified by pre-surgical functional mapping. Moreover, recent studies recommended incorporating cerebrovascular reactivity imaging into clinical fMRI to evaluate the risk of lesion-induced neurovascular uncoupling. However, a specialized clinical software that integrates the three complementary fMRI techniques and promptly outputs results to clinical PACS and surgical navigation system remains lacking. Here, we developed the Integrated fMRI for Clinical Research (IClinfMRI) software package to incorporate these advanced fMRI methods with streamlined processing and shortened the processing time for pre-surgical mapping and other clinical applications.
INTRODUCTION
Task-evoked functional MRI (fMRI) techniques have been applied to clinical management of neurological diseases 1, exemplified by pre-surgical functional mapping. Recently, resting-state (rs) fMRI has been showed helpful in clinical settings when patients have difficulties performing tasks 2,3. Moreover, recent studies recommended that incorporating cerebrovascular reactivity (CVR) imaging with hypercapnia challenges, such as a breath-hold (BH) task, into clinical fMRI protocol helps interpretation of results by indicating the risk of lesion-induced neurovascular uncoupling 4. Although aforementioned analyses for different fMRI modalities are feasible with existing research software, a specialized software that integrates the three complementary techniques and export the mapping results in DICOM format that are ready to export a PACS and surgical navigation system remains lacking. Here, we developed the Integrated fMRI for Clinical Research (IClinfMRI) software package to facilitate clinical fMRI researches with the applicability to pre-surgical planning.METHODS
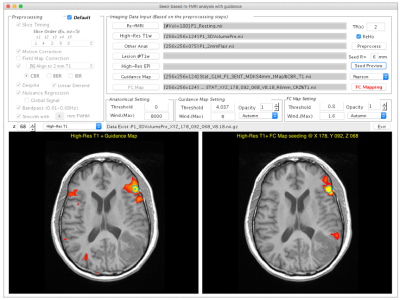
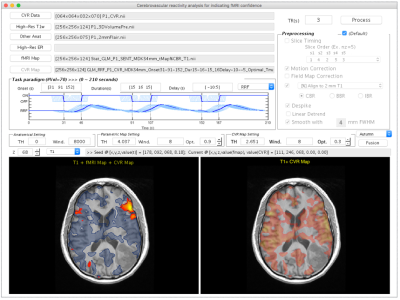
The IClinfMRI software was developed with a modular design that includes five functionalities: DICOM Import, Task fMRI, Resting-state fMRI, CVR mapping, and fMRI to PACS module. It was built upon in-house scripts on the MATLAB platform and calls functions in free copyright software, dcm2nii, AFNI, and SPM. In DICOM Import module, DICOM images in a file folder are converted to NIfTI files and organized by imaging series. In Task fMRI module, task-fMRI data is analyzed using the general linear (GLM) model. In Resting-state fMRI module, we designed a double-panel GUI to support the interactive rs-fMRI mapping while visualizing a guidance map. The guidance map can be an anatomical image, a parametric activation map, or a regional homogeneity (ReHo) map constrained by functional anatomy based on Meta-analysis 5 (RH+MA) provided by our software. In CVR mapping module, both hemodynamic and respiratory response function are available to generate CVR maps by using the GLM with an adjustable series of multiple time delays 6-8. Furthermore, special visualization is designed to fuse both fMRI and CVR maps on anatomical images for integrating results into a single presentation. In fMRI to PACS module, mapping results are converted to both color-coded and gray-scale overlays on structural images as DICOM images, respectively, for exporting to clinical PACS and surgical navigation system. Analyses of a 53-year-old glioma patient was used to illustrate the utility of IClinfMRI. The fMRI signal was acquired using GE-EPI sequence (TR/TE/FA=2000 ms/25 ms/90°, voxel size=3.75×3.75×4 mm3) on a 3T clinical scanner. The acquisition parameter of BH-MRI resembled that of fMRI, except that TR was 3000-ms. A total of 120, 180, 70 image volumes were obtained from language task-fMRI, rs-fMRI, and BH-MRI, respectively. The data were processed using with the default procedures in IClinfMRI.RESULTS
Typing “IClinfMRI” in the MATLAB command window will make the main GUI appear (Fig. 1). Figure 2 demonstrates the GUI of the Resting-state fMRI module. Both rs-fMRI and high-resolution T1w data are required, whereas the rest of three are optional inputs. Users can define a seed by left-clicking the mouse on imaging panels, and adjust the parameters related to seed-based rs-functional connectivity (FC) mapping. In the illustrated case, a seed (the turquoise dot with circular contour) was positioned on a point with peak t value of task-fMRI activation to detect connectivity in the Wernicke’s area near the lesion. After the processing toggled by “FC Mapping” button, the rs-FC z-map was displayed in the bottom-right panel. In this particular study, significant speech-fMRI activations (t > 4.04, uncorrected p < 10-4) were detected in bilateral frontal areas and right temporal/parietal area, but not left temporal/parietal area or the Wernicke’s area. The rs-fMRI was helpful in that a clear language network between left frontal and temporal/parietal areas were detected (z ≥ 0.8). Figure 5 shows the GUI of CVR Mapping module. After the processing, CVR map overlaying on structural images is automatically displayed and the “Fusion” button is enabled to fuse the CVR map to the pre-selected fMRI results. A markedly diminished ipsilateral CVR in areas within/near the tumor, which indicated NVU potentials and risk of false negative results in fMRI.CONCLUSION
IClinfMRI is a research software that is designed for translation to clinical practices by integrating advanced fMRI techniques and implementing data management/visulization strategies. With this tool, users can easily analyze task-fMRI, rs-fMRI and CVR mapping in patient’s individual space and integrate the information obtained. Moreover, our software supports to convert any parametric mapping results in NIfTI format to DICOM format for transferring to PACS. Here, we presented the IClinfMRI software to incorporate advanced fMRI methods with streamlined processing and shortened the processing time for pre-surgical mapping and other clinical applications.Acknowledgements
No acknowledgement found.References
1. Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci. 2006;7(9):732–744. doi:10.1038/nrn1929.
2. Hart MG, Price SJ, Suckling J. Functional connectivity networks for preoperative brain mapping in neurosurgery. J Neurosurg. August 2016:1–10. doi:10.3171/2016.6.JNS1662.
3. Mitchell TJ, Hacker CD, Breshears JD, et al. A novel data-driven approach to preoperative mapping of functional cortex using resting-state functional magnetic resonance imaging. Neurosurgery. 2013;73(6):969–82–discussion982–3. doi:10.1227/NEU.0000000000000141.
4. Pak RW, Hadjiabadi DH, Senarathna J, et al. Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors. J Cereb Blood Flow Metab. January 2017:271678X17707398. doi:10.1177/0271678X17707398.
5. Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665–670. doi:10.1038/nmeth.1635.
6. Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40(2):644–654. doi:10.1016/j.neuroimage.2007.11.059.
7. Pillai JJ, Zacà D. Comparison of BOLD cerebrovascular reactivity mapping and DSC MR perfusion imaging for prediction of neurovascular uncoupling potential in brain tumors. Technol Cancer Res Treat. 2012;11(4):361–374. doi:10.7785/tcrt.2012.500284.
8. Jahanian H, Christen T, Moseley ME, et al. Measuring vascular reactivity with resting-state blood oxygenation level-dependent (BOLD) signal fluctuations: A potential alternative to the breath-holding challenge? J Cereb Blood Flow Metab. January 2016:271678X16670921. doi:10.1177/0271678X16670921.
Figures