2829
Pseudo-CT generation from 3D multi-echo gradient-echo MRI1Medical Physics Unit, McGill University, Montreal, QC, Canada, 2Biomedical Engineering, McGill University, Montreal, QC, Canada, 3Research Institute of the McGill University Health Centre, Montreal, QC, Canada
Synopsis
MRI-based treatment planning in radiotherapy is limited by the lack of electron density information and by the difficulty to differentiate air from bone regions. A completely automatic method to produce a pseudo-CT from a 3D gradient-echo dataset through voxel-wise assignment of computed tomography (CT) numbers (Hounsfield unit (HU)) was developed. The HU assignment is based on a combination of relative fat and water content and magnetic susceptibility estimates. The proposed method avoids registration errors and allows for HU variability in each tissue class. An improved quantitative susceptibility mapping algorithm for regions with large susceptibility and negligible signal is also presented.
Introduction
Differentiation of air and bone regions in MRI is challenging. This is a limitation of MRI-based treatment planning in radiotherapy and a challenge for attenuation correction in PET-MR. Quantitative susceptibility mapping (QSM) can be used to separate bone from air based on their magnetic properties1. This work presents an algorithm for the automatic segmentation of bone, air, and soft tissue from a 3D gradient-echo MRI dataset, using a combination of magnitude images, QSM, and fat/water separation. The QSM technique is designed to map large susceptibility variations in regions with negligible signal. A voxel-wise assignment of computed tomography (CT) image values (Hounsfield units (HU)) based on the segmentation is also proposed, to create a pseudo-CT image.Methods
A 3D dataset was acquired with a multi-echo gradient-echo sequence with monopolar readout gradients in three healthy volunteers (3 T, TR=17.66 ms, bandwidth=953 Hz/pixel, ∆TE=2.4 ms, 1.3-mm isotropic resolution, flip angle=15°, number of echoes=3). Parallel imaging (SENSE, R=1.5) was used. The sequence was run twice (with TE1=1.5 ms and 2.7 ms, respectively). Data were combined by alternating echoes (shortest to longest TE) into a final dataset with six echoes and effective ∆TE=1.2 ms.
Fat/water separation was performed using a combination of the 3-point Dixon technique2 and the iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) algorithm3 with a multi-peak fat spectrum. The fat and water signal fraction maps were produced2. Binary fat and water masks were also produced by thresholding the respective signal fraction maps at 50%.
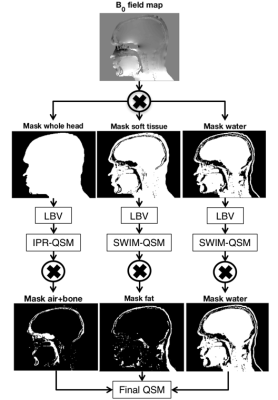
3D QSM was performed with a modified version of the iterative phase replacement algorithm (IPR-QSM)4, resulting in susceptibility estimates in soft tissue, bone, and air. Three masks were created using adaptive thresholding on magnitude images at TE1, respectively identifying the whole object (above background), soft tissue only, and air+bone. IPR-QSM was performed on the B0 field map from IDEAL, after quality-guided phase unwrapping5. A streaking correction was included to reduce artifacts from large susceptibility structures6,7. This streaking correction consisted of three-level background removal with the Laplacian boundary value (LBV) algorithm8,9 (Figure 1). The first level was a background removal step on the whole object mask, followed by IPR-QSM. The second level was performed on soft tissue only, followed by iterative susceptibility weighted imaging and mapping (SWIM) QSM10. The third and final level was performed using SWIM QSM on the water mask only. The final susceptibility map was a combination of these three steps.
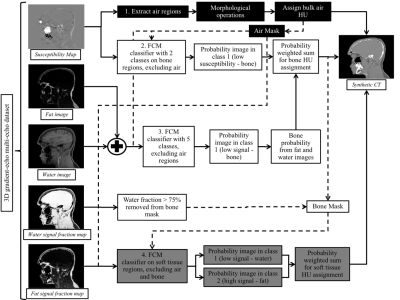
The pseudo-CT was produced using a segmentation algorithm based on fuzzy c-means (FCM) clustering and adaptive thresholding (Figure 2). Voxels classified as air were assigned a bulk HU of -1000. The voxel-based HU assignment in bone and soft tissue was performed using a probability-weighted sum:
$$sCT =P_b*1500+P_s*30+P_f*-100+P_w*30$$
Pb, Ps, Pf and Pw correspond to the probability of a voxel to be part of the bone, soft tissue, fat, and/or water classes, respectively.
Results
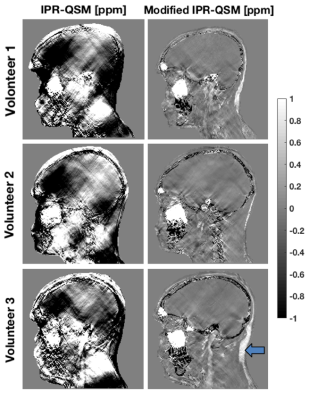
The
modifications to the IPR-QSM algorithm reduced streaking artifacts in the low
contrast regions compared to the original implementation. Notably, this
resulted in correct estimation of the paramagnetic susceptibility of fat (Figure
3). The susceptibility of cortical bone in the skull, which should be
diamagnetic, was mostly recovered by both QSM algorithms, but important
artifacts remained unresolved. The segmentation algorithm described in this
work mapped the skull properly despite limitations in the IPR-QSM values. The
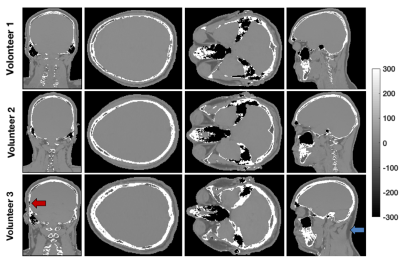
pseudo-CT results in the volunteers are shown in Figure 4. The skull, spine, frontal sinus, maxillary
sinus, mastoid sinus, ethmoid sinus, sphenoid sinus and teeth can be clearly distinguished.
Discussion and Conclusion
Differentiation of air and bone structures is possible using IPR-QSM in some regions, but the limitations of this technique prevent the accurate identification of the cortical bone in the skull, consistent with previous work1. The segmentation algorithm presented in this work resulted in an improved differentiation of air and bone compared to IPR-QSM only. The proposed HU assignment method allowed variations in each tissue class, as opposed to bulk HU assignment approaches proposed in the literature11. This completely automatic technique is fast and based on a commercially available pulse sequence. Finally, the method avoids registration errors typical of multi-sequence techniques12,13 and does not rely on a priori information, such as manual segmentation of bone12 or an atlas, making it suitable for nonstandard geometry. The combination of all six echoes into a single acquisition is under evaluation. Evaluation and validation in patients in ongoing.Acknowledgements
Fonds de Recherche Québec – Nature et Technologies, NSERC, Montreal General Hospital Foundation, NSERC-CREATE MPRTN grant 432290, Guillaume Gilbert (Philips Healthcare), Cornell MRI Research lab (MEDI toolbox), Sagar Buch and E. Mark Haacke for their assistance with the IPR algorithm and the developers of the ISMRM fat water toolbox (http://www.ismrm.org/workshops/FatWater12/ data.htm).References
1. E. Dixon, D. L. Thomas, A. Barnes, and K. Shmueli. Evaluation of air / bone segmentation using susceptibility-based imaging methods. In Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, Hawaii, USA, 2017. Abstract 3679.
2. J. Berglund, L. Johansson, H. Ahlström, and J. Kullberg. Three-point Dixon method enables whole-body water and fat imaging of obese subjects. Magn. Reson. Med., vol.63, no.6, pp.1659–1668, 2010.
3. S. B. Reeder, C. A. McKenzie, A. R. Pineda, H. Yu, A. Shimakawa, A. C. Brau, B. A. Hargreaves, G. E. Gold, and J. H. Brittain. Water-fat separation with IDEAL gradient-echo imaging. J. Magn. Reson. Imaging, vol.25, no.3, pp.644–652, 2007.
4. S. Buch, S. Liu, Y. Ye, Y. C. N. Cheng, J. Neelavalli, and E. M. Haacke. Susceptibility mapping of air, bone, and calcium in the head. Magn. Reson. Med., vol.73, no.6, pp.2185–2194, 2015.
5. V. Fortier and I. R. Levesque. (in press) Phase Processing for Quantitative Susceptibility Mapping of Regions With Large Susceptibility and Lack of Signal. Magn. Reson. Med., DOI 10.1002/mrm.26989, 2017.
6. H. Sun, M. Kate, L. C. Gioia, D. J. Emery, K. Butcher, and A. H. Wilman. Quantitative Susceptibility Mapping Using a Superposed Dipole Inversion Method: Application to Intracranial Hemorrhage. Magn. Reson. Med., vol.76, no.3, pp.781–791, 2015.
7. R. Sato, T. Shirai, Y. Taniguchi, T. Murase, A. Kuratani, T. Ueda, T. Tsuneki, Y. Bito, and H. Ochi. Quantitative susceptibility mapping with separate calculation in water and fat regions. In Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, Hawaii, USA, 2017. Abstract 3652.
8. D. Zhou, T. Liu, P. Spincemaille, and Y. Wang. Background field removal by solving the Laplacian boundary value problem. NMR Biomed., vol.27, no.3, pp.312–319, 2014.
9. Cornell MRI Research Lab. Quantitative Susceptibility Mapping - MEDI toolbox. 2013. [Online]. http://weill.cornell.edu/mri/pages/qsm.html.
10. J. Tang, S. Liu, J. Neelavalli, Y. C. N. Cheng, S. Buch, and E. M. Haacke. Improving susceptibility mapping using a threshold-based K-space/image domain iterative reconstruction approach. Magn. Reson. Med., vol.69, no.5, pp.1396–1407, 2013.
11. J. Wen, B. Cai, M. Gach, O. Green, C. Tsien, J. Huang, S. Mutic, and D. Yablonskiy. Tissue Relaxometry Defined ( TRD ) pseudo-CT imaging based on a single multi-gradient-echo MRI scan. In Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, Hawaii, USA, 2017. Abstract 4040.
12. W. Zheng, J. P. Kim, M. Kadbi, B. Movsas, I. J. Chetty, and C. K. Glide-Hurst. Magnetic Resonance-Based Automatic Air Segmentation for Generation of Synthetic Computed Tomography Scans in the Head Region. Int. J. Radiat. Oncol. Biol. Phys., vol.93, no.3, pp.497–506, 2015.
13. S.-H. Hsu, Y. Cao, K. Huang, M. Feng, and J. M. Balter. Investigation of a method for generating synthetic CT models from MRI scans of the head and neck for radiation therapy. Phys. Med. Biol., vol.48, pp.1–6, 2013.
Figures