2826
DCE-MRI Perfusion Analysis with L1-Norm Spatial Regularization1The Czech Academy of Sciences, Institute of Information Theory and Automation, Prague, Czech Republic, 2Department of Telecommunications, Brno University of Technology, Brno, Czech Republic, 3Masaryk Memorial Cancer Institute, Brno, Czech Republic, 4The Czech Academy of Sciences, Institute of Scientific Instruments, Brno, Czech Republic
Synopsis
DCE-MRI perfusion analysis suffers from low reliability, especially when 2nd-generation pharmacokinetic models are used to estimate perfusion parameter maps (voxel-by-voxel estimation) in low SNR conditions. These models provide estimates of plasma flow and capillary permeability in addition to the commonly used parameters Ktrans, kep. This contribution presents a method for estimation of perfusion maps using the tissue homogeneity model with incorporated spatial regularization in the form of total variation. The algorithm is based on the proximal minimization methods well established in image reconstruction problems. The use of state-of-the-art minimization and image regularization techniques stabilizes the estimates of perfusion parameter maps and keeps the computational demands low.
Introduction
Dynamic contrast-enhanced (DCE) MRI becomes an established tool to obtain information about tissue perfusion and vessel permeability. It is beneficial, when this information is represented by a set of physiological parameters (plasma flow, vascular volume, capillary permeability…) for each voxel in the region of interest, which gives so-called perfusion parameter maps. To get the parameter maps, the concentration-time curve of each voxel is fitted by a pharmacokinetic model. This curve-fitting is not trivial because of model nonlinearity, high dimensionality, insufficient temporal sampling and low signal-to-noise ratio (SNR), or uncertainties in either the model or measurement. This results in bias and uncertainty in the estimates or wrong estimates because of local minima1.
A possible solution to overcome these problems is to use additional a priori information. Here, the nonlinear least squares curve-fitting problem is extended by a spatial regularization term (image prior) in the form of a total variation (TV), well known in image processing2. This non-differentiable L1-norm regularizer preserves piecewise smoothness of images. Other authors1,3 have used differentiable spatial regularizers: Tikhonov, Lp norm for $$$p\in(1,2\rangle$$$, respectively, which tend to blur the solution. The main reason to use differentiable regularizers is to keep minimization tractable. We propose a method, which can handle state-of-the-art non-differentiable regularizers and reduces memory demands.
Method
The problem is formulated as a maximum a posteriori probability estimation, where the prior is in the form of a sum of 5 TV regularizations (sum of L1 norms of the magnitude of image gradient). The data term is in the form of nonlinear least squares. The pharmacokinetic model is a time-domain convolution of a known arterial input function (AIF) with the tissue homogeneity model4 (TH). Its five parameters including bolus arrival time are to be estimated per each voxel. The convolution is evaluated in the Fourier domain because the TH model has no closed-form expression in the time domain.
The minimization is solved using the proximal gradient method5 to treat non-differentiability of the regularizer. The adopted algorithm iteratively repeats the following two steps until convergence: 1) perform one steepest descent step per each voxel, 2) evaluate the proximal operator to step 1). The evaluation of the proximal operator is in the form of the five image denoising problems, with different noise variances in each voxel, regularized by the used total variation. This sub-problem is solved by the Chambolle-Pock proximal algorithm6 adapted to the case with non-uniform noise variances. The sub-algorithm iteratively updates: 2a) per-voxel projection onto a unit circle in the domain of the image gradients, 2b) simple voxel-wise operations on the reconstructed image from 2a), 3b) voxel-wise update of perfusion map from 2b) using the solution from the previous iteration.
Evaluation and Discussion
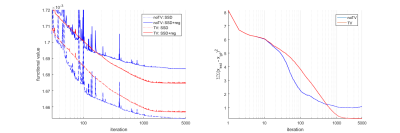
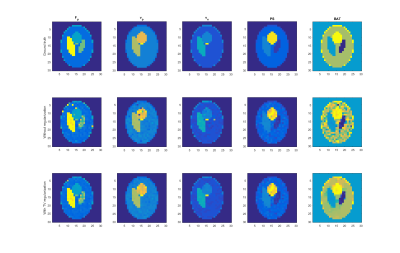
The algorithm was evaluated on simulated data based on the Shepp-Logan phantom and literature perfusion parameters and AIF (Figure 2 – top). The algorithm was qualitatively compared with its version with no TV regularization (Figure 2 – row 2, 3). The convergence properties (Figure 1) were also investigated. The regularization stabilized the estimation process (Figure 2 – column “BAT”) and reduced the number of outliers.
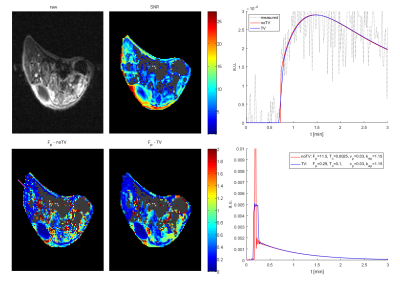
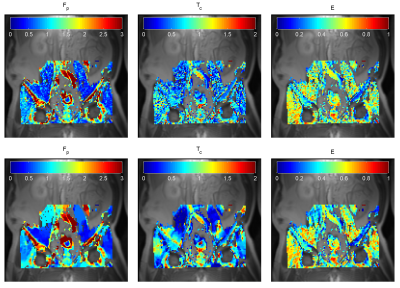
The potential of the algorithm to reduce outliers (no. of local minima) was also tested on a pre-clinical dataset (Figure 3) of BALB/C mice after subcutaneous implantation of tumor cells (CT26), approved by the National Animal Research Authority, and on an anonymized clinical dataset of a metastatic renal cell carcinoma in the pelvic area (Figure 4). In both cases, the readability of the estimated maps has increased, but the accuracy and precision, of the estimates need further investigation to be described quantitatively.
Conclusion
The presented method shows a possible option to incorporate spatial regularization into the problem of the voxel-by-voxel perfusion parameter estimation. The spatial regularization allows us to use models with higher number of parameters in low SNR conditions. Without the regularization, the minimization is unstable (high uncertainty, local minima, …). The advantage of the proposed method is its voxel-wise character, except the computation of the image gradients. In comparison with alternatives1,3, the dimensionality (computational demands) did not depend on a support of the transform in the regularizer (the image gradients can be interchanged by e.g. wavelet transform) thus the algorithm can use many state-of-the-art non-differentiable priors known of the image processing community. Because the algorithm is derived for the problems in the form of weighted nonlinear least squares with spatial regularization, it can be adopted to other similar reconstruction problems by replacing the pharmacokinetic model.Acknowledgements
This work was supported by The Czech grant agency (GA16-13830S) and by MEYS - NPS I - LO1413.References
1. Kelm BM, Menze BH, Nix O, Zechmann CM, Hamprecht FA. Estimating Kinetic Parameter Maps From Dynamic Contrast-Enhanced MRI Using Spatial Prior Knowledge. IEEE Trans Med Imaging 2009;28:1534–47.
2. Rudin LI, Osher S, Fatemi E. Nonlinear total variation based noise removal algorithms. Phys D Nonlinear Phenom 1992;60:259–68.
3. Sommer JC, Gertheiss J, Schmid VJ. Spatially regularized estimation for the analysis of dynamic contrast-enhanced magnetic resonance imaging data. Stat Med 2014;33:1029–41.
4. Garpebring A, Ostlund N, Karlsson M. A Novel Estimation Method for Physiological Parameters in Dynamic Contrast-Enhanced MRI: Application of a Distributed Parameter Model Using Fourier-Domain Calculations. IEEE Trans Med Imaging 2009;28:1375–83.
5. Parikh N, Boyd S. Proximal Algorithms. Found Trends Optim 2013;1:123–231.
6. Chambolle A, Pock T. A first-order primal-dual algorithm for convex problems with applications to imaging. J Math Imaging Vis 2011;40:120–45.
Figures