2820
Impact of ICA-based denoising of ASL data in clinical settings1Acute Vascular Imaging Centre, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom, 2Laboratory of Experimental Stroke Research, Department of Surgery and Translational Medicine, Milan Center of Neuroscience, University of Milano Bicocca, Monza, Italy, 3Wellcome Centre for Integrative Neuroimaging, Oxford Centre for Functional MRI of the Brain, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 4Institute of Biomedical Engineering, Department of Engineering Science, University of Oxford, Oxford, United Kingdom
Synopsis
ASL data has a low signal to noise ratio (SNR) and is sensitive to motion. Independent component analysis (ICA) has been successfully applied to denoise similar quality data in fMRI. We explored the effects of using an ICA approach on ASL data acquired in two different clinical settings. Mean cerebral blood flow (CBF) values were identical pre- and post- ICA indicating good signal preservation. However, the variance of CBF and bolus arrival time measures was significantly reduced suggesting a reduction in noise. These results suggest that ICA based denoising represents a promising strategy to improve ASL data quality.
Introduction
In arterial spin labeling (ASL)1, a perfusion weighted image is achieved by subtracting a label image from a control image. This perfusion weighted image has an intrinsically low signal to noise ratio (SNR), and numerous measurements are required to achieve higher image quality2. Additionally the subtraction process makes this technique particularly susceptible to motion artefacts. This is of particular importance in clinical settings where noise levels and motion are greater3, and time constraints limit the scan duration and hence the number of measurements. Independent component analysis (ICA) is used to reliably separate signal from noise in functional MRI4, diffusion-weighted imaging5, and dynamic susceptibility contrast-MRI6 and has shown promising preliminary results when applied to ASL data acquired in animals7. The aim of this work was to evaluate the effects of ICA denoising in ASL data obtained in acute and chronic settings.
Methods
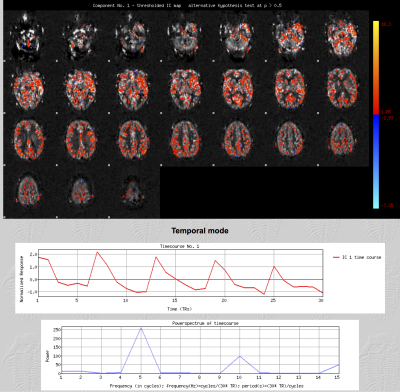
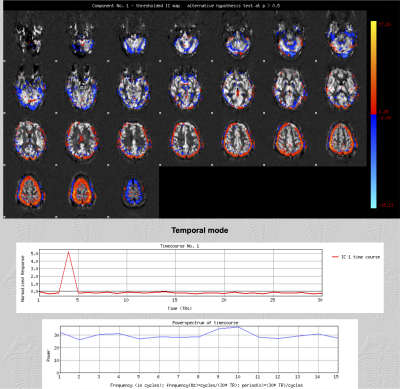
25 ASL datasets (pcASL, single EPI readout, TR/TE 5386/14ms, 3.4x3.4x4.5mm, 24 slices using a matrix size 64x64. Alternating control and tag pairs were acquired after 1.8 s of labelling at 6 different post labelling delays: 300, 700, 1100, 1500, 1900 2300 ms, each one repeated 5 times; acquisition time= 4'30'') were collected along with a high resolution T1-weighted structural imaging (magnetization prepared rapid acquisition gradient echo, 1.8×1.8×1.0 mm, FoV=228 mm, TR=2040 ms, TE=4.55 ms, and acquisition time=3'58'') from 8 consecutive chronic patients (cirrhosis without encephalopathy) and 10 consecutive acute stroke patients. All scans were acquired using a 3.0T Siemens Verio scanner (Siemens Healthcare, Erlangen, Germany). Each 4D pre-processed and subtracted dataset entered single-subject spatial-ICA decomposition with automatic dimensionality estimation using Multivariate Exploratory Linear Optimised Decomposition of Independent Components (MELODIC)8. Components were classified as signal (Fig.1) when at least one of the following characteristics was present: spatial maps consistent with vascular territories; a time course congruous with the post-labelling delays; and, most of the signal in the power spectrum at frequencies corresponding to the number of repetitions or its multiple. If a component had none of these characteristics, it was labelled as noise (Fig. 2) and regressed out of the data. Cerebral blood flow (CBF), bolus arrival time and their respective variance were estimated before and after artifactual component removal using a spatially regularised Bayesian inference method. Mean values in gray matter, white matter and CSF were compared using a paired t test. Additionally, for each ASL dataset acquired, 5 epochs comprised of 6 volumes (one for each post-labelling delay) were generated and gray matter perfusion was estimated in each epoch. A Bland Altman analysis was performed to determine the agreement between these estimates (before and after denoising) and the perfusion values derived using the whole dataset. In all stages, tools from the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL 5.0, Oxford, UK; www.fmrib.ox.ac.uk/fsl) were used.
Results
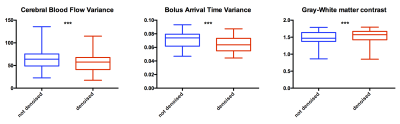
No meaningful changes were found at a group level in either gray or white matter CBF/arrival times. CBF and arrival time variance were both significantly reduced after regressing noise components (mean diff. 14.5% p<0.001 and 10% p<0.001, respectively; see Fig. 3). The ratio between gray matter and white matter perfusion was significantly improved after data denoising (mean diff 3%, p<0.01, see Fig. 3). Agreement between estimation of gray matter perfusion obtained from each of the epochs and the whole ASL dataset was higher in denoised data (before denoising bias -1.63 SD 4.79, after denoising bias -1.354 SD 3.977 ).
Discussion
The inherently low SNR and motion sensitivity of ASL data makes denoising essential for robust perfusion quantification. The results of this study demonstrate the potential of using an ICA based denoising approach for ASL. The decrease in CBF/arrival time variance in the denoised datasets suggests that regressing artifactual components leads to the removal of actual noise from the data. Importantly, the absence of a meaningful effect on the mean CBF and on arrival times at a group level suggests also signal preservation. Increasing the number of measurements (repeats) improves the SNR2. ICA denoising was shown to generate similar results as the agreement between epochs and the whole datasets was higher after denoising. The higher gray/white matter perfusion ratio present in denoised data also supports this conclusion, as it is closer to expected literature values9.
Conclusion
Our results suggest that ICA can be used to separate signal from noise in ASL data. The removal of artifactual components to denoise ASL data represents a promising strategy to increase the quality of the data obtained without having to increase acquisition duration.
Acknowledgements
We wish to acknowledge the facilities provided by the Oxford Acute Vascular Imaging Centre and the staff of the Oxford Acute Stroke Programme. This study was supported by the National Institute for Health Research Oxford Biomedical Research Centre Programme, the National Institute for Health Research Clinical Research Network, the Dunhill Medical Trust [grant number: OSRP1/1006] and the Centre of Excellence for Personalized Healthcare funded by the Wellcome Trust and Engineering and Physical Sciences Research Council under grant number WT088877/Z/09/Z.References
1. Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magnetic Resonance in Medicine. 1992;23:37-45 2. Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended implementation of arterial spin-labeled perfusion mri for clinical applications: A consensus of the ismrm perfusion study group and the european consortium for asl in dementia. Magnetic Resonance in Medicine. 2015;73:spcone-spcone 3. Carone D, Licenik R, Suri S, Griffanti L, Filippini N, Kennedy J. Impact of automated ica-based denoising of fmri data in acute stroke patients. NeuroImage. Clinical. 2017;16:23-31 4. Thomas CG, Harshman RA, Menon RS. Noise reduction in bold-based fmri using component analysis. NeuroImage. 2002;17:1521-1537 5. Arfanakis K, Cordes D, Haughton VM, Carew JD, Meyerand ME. Independent component analysis applied to diffusion tensor mri. Magn Reson Med. 2002;47:354-363 6. Calamante F, Morup M, Hansen LK. Defining a local arterial input function for perfusion mri using independent component analysis. Magnetic Resonance in Medicine. 2004;52:789-797 7. Wells JA, Thomas DL, King MD, Connelly A, Lythgoe MF, Calamante F. Reduction of errors in asl cerebral perfusion and arterial transit time maps using image de-noising. Magn Reson Med. 2010;64:715-724 8. Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE transactions on medical imaging. 2004;23:137-152 9. Bristow MS, Simon JE, Brown RA, Eliasziw M, Hill MD, Coutts SB, et al. Mr perfusion and diffusion in acute ischemic stroke: Human gray and white matter have different thresholds for infarction. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:1280-1287Figures