2814
Compressed Sensing 3D Double Inversion Recovery (DIR) in the Brain1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 2Department of Radiology, University Hospital (CHUV), Lausanne, Switzerland, 3LTS5, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 4Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Double Inversion Recovery (DIR) provides a clinical valuable contrast, especially to study gray matter tissue alterations. However, long acquisition times hinder its use in clinical routine. Assuming that the inherent sparsity of the contrast is well suited for compressed sensing, we tested an incoherently undersampled 3D variable-flip-angle fast spin echo sequence with subsequent compressed sensing reconstruction. The reconstructed fourfold accelerated images exhibit image quality similar to both the clinical standard (twofold GRAPPA-accelerated) and to the fully sampled acquisition. The proposed sequence with ~4 min acquisition time may allow a more frequent use of DIR in clinical routine.
Introduction
Double Inversion Recovery (DIR) imaging, providing a contrast where cerebrospinal fluid (CSF) and white matter (WM) are nulled, is used in neurological applications mostly to study gray matter (GM) and to detect intra-cortical lesions in multiple sclerosis1. However, it requires relatively long repetition times to allow for T1 relaxation after the application of two inversion pulses, resulting in long acquisition times. Therefore, DIR is rarely used in clinical routine. Since the signal of WM and CSF is nulled, DIR images are inherently sparse and may be a good candidate for compressed sensing2. Here we introduce an incoherently undersampled 3D variable-flip-angle fast spin echo (SPACE) sequence3,4 for DIR imaging using a conventional compressed sensing reconstruction5.Methods
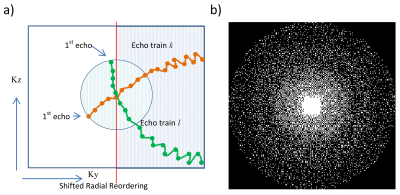
Undersampled k-spaces of three healthy subjects were acquired after obtaining written consent using a prototype 3D SPACE sequence at 3T (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) equipped with a standard 64-channel head/neck coil. Acceleration factors with CS were twofold (TA = 6:39 min), threefold (TA = 5:02 min), fourfold (TA = 4:09 min) and tenfold (TA = 2:09 min). Relevant sequence parameters were 1 x 1 x 1.2 mm3 voxel-size, 256 x 256 x 176 matrix, TI = 3000/450 ms, TR = 7.5 s, TE = 326 ms and TurboFactor = 256. The gradient pattern was applied to perform a shifted radial trajectory through the incoherently (Poisson disk) sampled k-space (Figure 1). For comparison, a conventional DIR sequence was used to acquire fully sampled (TA=10:47min) and twofold GRAPPA accelerated (TA=6:17min) DIR images using sequence parameters as similar as possible to the CS acquisition. The k-space trajectory of these reference acquisitions was a conventional linear gradient pattern. Since sparsity (and hence CS performance) typically increases with larger matrix size, an additional dataset with high resolution ( (0.8 mm)3 isotropic voxel-size, 320 x 320 x 240 matrix) was acquired for each subject with an 6.25-fold acceleration resulting in the same acquisition time as a conventional GRAPPA reconstruction at lower resolution (TA = 6:17 min).
The incoherently undersampled data was reconstructed using a compressed sensing reconstruction that minimizes the least-squares difference between the estimated image x and the undersampled k-space y and enforces sparsity in the wavelet domain:
$$\underset{x}{argmin}\frac{1}{2}{\vert\vert}Ax-y{\vert\vert}_{2}^2+\lambda\vert\phi(x)\vert_{1}$$
with A denoting the linear forward sampling operator that incorporates coil sensitivities, Fourier transform and undersampling, and Φ denoting the Wavelet transform. The regularization parameter λ was manually tuned for each undersampling factor (2-fold=0.0015, 3-fold=0.0015, 4-fold=0.002, 6.25-fold=0.009, 10-fold=0.002 ) and 20 iterations were performed.The resulting images were visually (criteria: noise, blur, regularization artifacts) compared.
Results
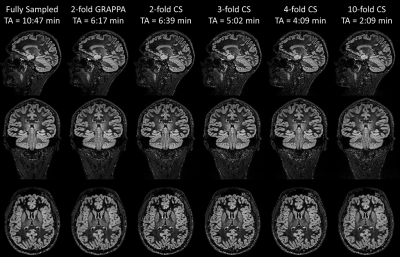
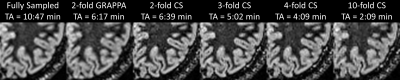
Figure 2 shows the acquired DIR images from one example volunteer. When comparing the GRAPPA-accelerated to the fully sampled DIR image, typical noise-amplification in conjunction with SNR loss due to undersampling can be observed. In contrast, the incoherently undersampled acquisition with compressed sensing reconstruction is less blurred in comparison to both reference images (fully sampled and GRAPPA), which may be linked to the k-space trajectory (shifted radial vs. linear), resulting in different T2-blurring. With increasing acceleration factors, the CS-reconstructed images exhibit stronger regularization artifacts (typical wavelet artifacts). Figure 3 shows a zoom into the left occipital lobe in an axial orientation. Qualitatively, the compressed sensing reconstructions appear superior to the fully sampled and GRAPPA images when considering blurring as quality criteria. However, detection of small pathology may be more difficult due to the stronger regularization at the highest acceleration (10-fold acceleration, TA=2:09min).
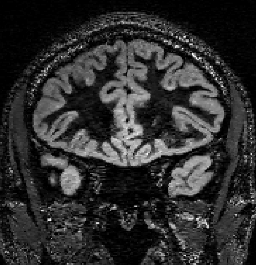
Figure 4 shows the entire volume of a high-resolution dataset in a coronal view. The small (0.8mm)3 voxel size (0.512µl) and high acceleration (6.25-fold) results in increased noise and regularization artifacts in comparison to a voxel size of 1.2µl. However, the higher resolution may be beneficial for the detection of smaller lesions.
Discussion & Conclusion
The proposed method demonstrates that DIR is a good candidate for acceleration using compressed sensing. Especially the reduced T2 blurring caused by a different k-space trajectory seems to improve the apparent resolution. With a 4-fold acceleration, DIR images with a similar or even better quality compared to a fully sampled reference were acquired in 4:09 min.
Future studies should investigate if the employed regularization in the reconstruction affects the detection rate of GM alterations, especially lesions in multiple sclerosis patients. Furthermore, the tradeoff between undersampling and image quality needs to be systematically studied in a clinical context.In conclusion, a proof of concept to accelerate 3D DIR acquisitions showed less blurring with an acceptable level of regularization artifacts, which may facilitate the use of DIR in clinically acceptable acquisition times (~4min).Acknowledgements
No acknowledgement found.References
- Geurts, Jeroen JG, et al. "Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging." Radiology 236.1 (2005): 254-260.
- Gho, Sung-Min, et al. "Three dimension double inversion recovery gray matter imaging using compressed sensing." Magnetic resonance imaging 28.10 (2010): 1395-1402.
- Li G, Zaitsev M, Büchert M, et al. „Improving the robustness of 3D turbo spin echo imaging to involuntary motion”. MAGMA. 28 (2014):329–345.
- Fritz J, Raithel E, Thawait GK, Gilson W, Papp DF. “Six-Fold Acceleration of High-Spatial Resolution 3D SPACE MRI of the Knee Through Incoherent k-Space Undersampling and Iterative Reconstruction-First Experience.” Invest Radiol. 51(6) (2016):400-9.
- Lustig, Michael, David Donoho, and John M. Pauly. "Sparse MRI: The application of compressed sensing for rapid MR imaging." Magnetic resonance in medicine 58.6 (2007): 1182-1195.
Figures