2800
Convolutional neural network segmentation of skeletal muscle NMR images1Mathematical Cybernetics, United Institute of Informatics Problems, Minsk, Belarus, 2NMR Laboratory, Institute of Myology, Paris, France, 3NMR Laboratory, CEA,DRF,IBFJ,MIRCen, Paris, France, 4Consultants for Research in Imaging and Spectroscopy, Tournai, Belgium
Synopsis
The purpose of this work was to investigate the ability of deep convolutional neural networks (CNN) to segment muscle groups in NMR images. To this end, we used lower limb scans of patients with different neuromuscular diseases and various levels of fatty infiltration. Thigh and leg muscle groups were first segmented manually and then used in the training and validation processes of the CNN. The mean Dice coefficient of the obtained segmentations was 0.9, demonstrating the effectiveness of the technique in automatically segmenting both healthy and pathological muscle groups.
INTRODUCTION
In neuromuscular diseases, quantitative NMR imaging provides ways to analyze muscle trophicity and structural changes. Muscles involvement being different from one pathology to another, studying muscles individually or at least by muscle groups is recommended for a proper characterization of the disease stages. This requires delineating the muscles in the images, a task usually performed by hand. Some attempts to tackle the automatic segmentation problem (1–3) were at least partly successful, but the question is still largely open. The aforementioned techniques relied on gray level differences between neighboring pixels, which is a very rudimentary descriptor of the local properties, and prior knowledge of the statistical location of the muscles (e.g. through registration of an atlas), which makes non-flexible and fallible anatomical models. Conversely, deep convolutional neural network (CNN) segmentation techniques do not require any prior selection of features, i.e. explicit descriptors of local image information, nor any explicit anatomical model (4). In this work, our purpose was to take advantage of the extensive learning capabilities of CNN and apply them to segment muscle groups in NMR images.METHOD
Method description: The CCN is composed of an encoder made of several convolutions filter banks, max-pooling and subsampling operator to produce sparse and multi-scale image representation. The decoder part is symmetrical, with the pooling layer replaced by up-sampling layer and a soft maximum classifier at the end. The decoder stage is responsible for producing multi-dimensional features for each pixel. The learning process consists in calculating the optimal encoder and decoder weights based on the training samples.
Data : all data were acquired on a 3T Siemens scanner and using a proton density weighted 3D Gradient-echo sequence with TR TE=2.75/3.95/5.15 ms with a pixel resolution of 1x1x5 mm3 and a 448x224x64 matrix size. We used only the out-of-phase volume at TE=3.95 ms where muscle fasciae are the most visible. The database was composed of 148 and 161 volumes, respectively covering the thighs and the legs of patients with various neuromuscular diseases. The targeted muscle groups were the Quadriceps, Hamstring, Triceps surae, Foot Extensor and Fibularis groups. We manually segmented 5 slices in every volume to provide annotations for the training and testing of the CNN.
Practical implementation: The training phase was performed with 2D slices of size (448x224) with their corresponding segmentation. We used both Keras and Theano Python libraries as well as cuDNN, a GPU-accelerated library of primitives for deep neural networks from NVidia. The training phase took 6 hours using an NVidia Titan X (Maxwell architecture, 3072 CUDA cores, 12 Gb of VRAM). For the testing part, the segmentation of one volume (64 slices) took less than 2s.
RESULTS
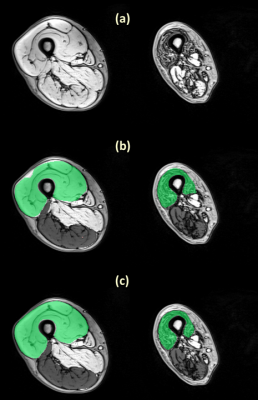
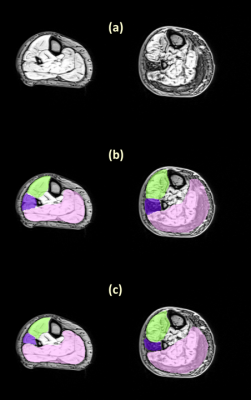
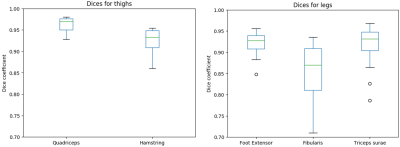
To evaluate the segmentation performance, we computed the Dice coefficient of the comparison between the manual and the automatic segmentation on a set of 70 slices for the thigh and 65 slices for the leg. Figure 1 and figure 2 show examples of segmentation obtained on thigh and leg muscle groups without fatty infiltration as well as diseased ones. The Dice coefficients are presented in figure 3, showing values above 0.9 on average.DISCUSSION
The results showed a good agreement between the manual and the fully automatic segmentation. The algorithm performance was lower for the Hamstring group, possibly due to its higher shape variability. The dice coefficient were also lower for leg muscle groups, probably due to their small size and high variability of shapes and appearances. The high Dice values on patients with neuro-muscular diseases and various degrees of muscle fatty infiltrations demonstrated the ability of the algorithm to handle such data, unlike comparable published work in which only automatic segmentation of healthy muscles was attempted (1–3). A method to address the problem of segmenting pathological muscles was presented in (5) , but it required some user input whereas our approach was fully automatic. However, the proposed method used only one volume out of a multi-echo NMR imaging sequence. Possible improvements could be achieved by taking the amplitude of the different volumes acquired at different TEs as an input for the algorithm.CONCLUSION
We presented and validated an automatic segmentation method for NMR images of healthy and pathological muscle groups based on the CNN framework. The results are promising and demonstrate the potential of CNN-based automatic approaches.Acknowledgements
No acknowledgement found.References
1. Baudin P, Azzabou N, Carlier P. Manifold-enhanced Segmentation through Random Walks on Linear Subspace Priors. BMVC, 2012:1–10.
2. Baudin PY, Azzabou N, Carlier PG, Paragios N. Prior knowledge, random walks and human skeletal muscle segmentation. MICCAI, 2012;15:569–76.
3. Andrews S, Hamarneh G, Yazdanpanah A, HajGhanbari B, Reid WD. Probabilistic multi-shape segmentation of knee extensor and flexor muscles. MICCAI, 2011;14:651–658.
4. Badrinarayanan V, Kendall A, Cipolla R. SegNet: A Deep Convolutional Encoder-Decoder Architecture for Image Segmentation. IEEE PAMI, 2017;39:2481–2495.
5. Baudin P, Beyeler M, Carlier PG, Scheidegger O. Fast delineation of calf muscles for quantitative MRI applications. ISMRM ,2017.
Figures