2762
Body Phantom with Prostate Mimic for Evaluation of Quantitative MRI1Center for Magnetic Resonance Research and Department of Radiology, University of Minnesota, Minneapolis, MN, United States, 2Imaging Standards Division, High Precision Devices, Inc., Boulder, CO, United States
Synopsis
A body phantom, containing a prostate mimic with traceable T1/T2/ADC standards, was designed and manufactured to assess acquisition-, system-, and RF coil- dependent variances of quantitative MRI parameters. In order to explore the potential of the phantom as a quality assurance tool, two phantoms were constructed and evaluated with two receive coil configurations across two scanners over a period of three weeks. It is demonstrated that this phantom is a useful prostate specific quality assurance tool and provide the information needed to harmonize results thus minimizing the impact of multiple dependencies on quantitative results.
Purpose
A multiparametric approach to magnetic resonance imaging (mpMRI) has become the standard for prostate cancer detection, staging, and monitoring. Clinical evaluation of mpMRI data has been standardized through the development of PI-RADS1. While PI-RADS has codified the qualitative assessment of mpMRI data, quantitative MRI (qMRI) in the form of predictive models2 have shown promise in cancer detection which better integrate the multiple measurements comprising an mpMRI study while being less susceptible experience dependent performance when using PI-RADS3,4. In order to disseminate qMRI methods developed at one center to others, it is important to understand the sources of variability in qMRI measurements to harmonize results and for quality assurance as systems are upgraded and new acquisition methods are introduced. Here we present an anthropomorphic prostate phantom designed to evaluate the stability and accuracy of multiple qMRI parameters with multiple RF coil configurations.Methods
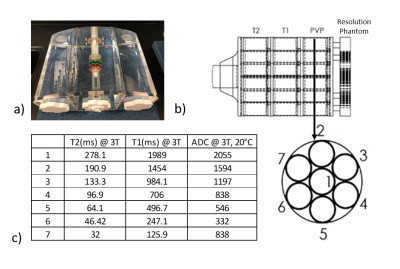
Phantom Details: Two identical phantoms were designed in collaboration with the manufacturer (High Precision Devices, Boulder, CO) and each was comprised of three sections: a center of approximately 7.5L and two side wings of approximately 4.3L each which attach to the central section to form a 16L anthropomorphic torso with overall dimensions of 40x19x30cm (WxHxL). The body of the phantoms were filled with saline providing body matched conductivity. Positioned in the middle of the central section is a prostate phantom mimic positioned slightly anteriorly based on previous in vivo measurements5. The prostate phantom contains a resolution plate along with three sets of seven vials, one set each for T1, T2, and ADC NIST6 standards representing a range of values relevant for prostate investigations (Figure 1). The prostate assembly can be freely rotated to allow the impact of receive and transmit field variations on qMRI parameters to be assessed. Multiple coil configurations can be investigated with the current design including surface arrays with or without endorectal coils (ERC). To accommodate ERC coils two different size tubes one for a solid-type and one for balloon-type ERC can be inserted into the phantom just posterior to the prostate assembly.
Experimental Evaluation: Experiments were conducted on two whole-body 3 T Siemens Prisma Trio systems (Siemens, Erlangen, Germany) using either a 32-element spine coil with 24-element body coil, or spine and body coils in combination with a 2-element solid endorectal coil.
More time efficient quantitative imaging protocols used in clinical practice were compared against gold standard methods such as variable inversion recovery (VIR) spin echo (SE)7 for T1 mapping and a multi-echo spin echo acquisition for T2 measurements using 12 echo times (SE_MC). The clinical protocols, which conformed to PIRADS and QIBA technical specifications where appropriate8, included: variable flip angle (VFA) T1 mapping (flip angles of 2,5,10, and 15 degrees), T2 mapping with multiple fast-spin-echo images (echo times of 30, 71, 107 and 144 ms), and diffusion weighted imaging (DWI) with a 2D selective RF pulse (i.e. ZOOMIT) (b-values of 0, 50, 400, 800, 1200). A standard DWI sequence with GRAPPA R2 enabled in the AP direction was also investigated for comparison with ZOOMIT.
The two manufactured body phantoms were scanned on one system to assess production consistency. Additional studies were carried out on both MRI systems across three weeks to examine inter- and intra- scanner dependent variability/repeatability.
Results
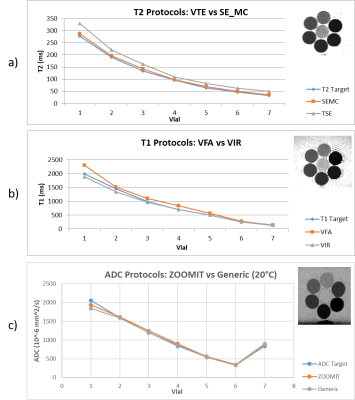
Sequence Evaluations: Significant variability in values across protocols is depicted in Figure 2. The VIR method is more accurate than the VFA, but the scan time required for the selected inversion times is in excess of 90 minutes and isn’t feasible for regular data acquisition. The fast-spin-echo protocol’s T2 values are consistently higher than the target T2 values by 20% or more. This is likely due to the production of a stimulated echo and B1+ profile dependencies. SE_MC values are more accurate, precise, and faster.
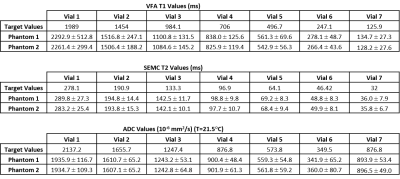
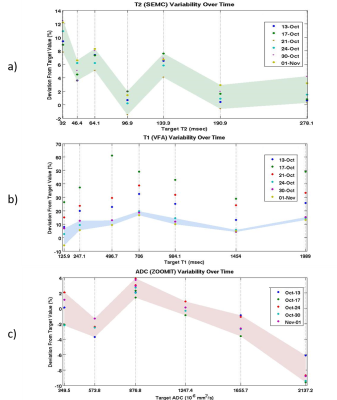
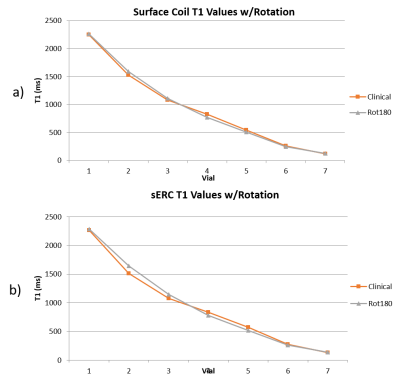
Hardware Evaluations: A phantom vs. phantom comparison of the two phantoms, Table 1, reveals excellent agreement in measured values. Figure 3 depicts temporal variability for a single scanner using a surface coil. Two scanners using the same coil configuration and phantom were evaluated with no significant variations in the acquired values. Shown in Figure 4 are the differences between coil configurations and the resulting effects of rotation on the measured values due to multiple effects.
Conclusion/Discussion
The use of non-anthropomorphic phantoms can lead to misleading results9,10. It is demonstrated that this phantom is a useful quality assurance tool for prostate specific imaging and, even more interesting, a powerful tool for expanding the generalization of quantitative imaging methods and diagnostics by providing the information needed to harmonize results thus minimizing the impact of multiple dependencies on specific quantitative results.Acknowledgements
Supported by: NCI R01 CA155268, NIBIB P41 EB015894, DOD W81XWH-15-1-0477, and the Minnesota Research Evaluation and Commercialization Hub (MN-REACH)References
1. Spektor, M., M. Mathur, and J.C. Weinreb, Standards for MRI reporting-the evolution to PI-RADS v 2.0. Transl Androl Urol, 2017. 6(3): p. 355-367.
2. Metzger, G.J., et al., Detection of Prostate Cancer: Quantitative Multiparametric MR Imaging Models Developed Using Registered Correlative Histopathology. Radiology, 2016. 279(3): p. 805-16.
3. Barentsz, J.O., et al., ESUR prostate MR guidelines 2012. Eur Radiol, 2012. 22(4): p. 746-57.
4. Rosenkrantz, A.B., et al., Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology, 2013. 269(2): p. 482-92.
5. Metzger, G.J., et al., Local B1+ shimming for prostate imaging with transceiver arrays at 7T based on subject-dependent transmit phase measurements. Magn Reson Med, 2008. 59(2): p. 396-409.
6. Keenan, K.E., et al. Comparison of T1 measurements using ISMRM/NIST system phantom. in ISMRM 24th Annual Meeting. 2016. Singapore.
7. Weinreb, J.C., et al., PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol, 2016. 69(1): p. 16-40.
8. Crawley, A.P., M.L. Wood, and R.M. Henkelman, Elimination of transverse coherences in FLASH MRI. Magn Reson Med, 1988. 8(3): p. 248-60.
9. Freed, M., et al., An anthropomorphic phantom for quantitative evaluation of breast MRI. Med Phys, 2011. 38(2): p. 743-53.
10. Keenan, K.E., et al., Design of a breast phantom for quantitative MRI. J Magn Reson Imaging, 2016. 44(3): p. 610-9. 12. Wagner, F., et al., Temperature and concentration calibration of aqueous polyvinylpyrrolidone (PVP) solutions for isotropic diffusion MRI phantoms. PLoS One, 2017. 12(6): p. e0179276.
11. Wagner, F., et al., Temperature and concentration calibration of aqueous polyvinylpyrrolidone (PVP) solutions for isotropic diffusion MRI phantoms. PLoS One, 2017. 12(6): p. e0179276.
Figures