2761
Lateralization of Temporal Lobe Epilepsy Using Multimodal Neuroimaging Models1Research Center for Molecular and Cellular Imaging, Tehran University of Medical Sciences, Tehran, Iran (Islamic Republic of), 2Clinical Neurosciences, Spectrum Health Medical Group, Grand Rapids, MI, United States, 3Radiology and Research Administration, Henry Ford Health System, Detroit, MI, United States, 4Control and Intelligent Processing Center of Excellence (CIPCE), School of Electrical and Computer, University of Tehran, Tehran, Tehran, Iran (Islamic Republic of)
Synopsis
In this work, multivariate response-driven lateralization models were developed using MRI, DTI, and SPECT attributes and logistic regression, to determine the side of epileptogenicity in TLE patients. The proposed response models were capable of handling missing data points using imputation of missing attributes by their mean values measured on a control cohort. Additionally, the proposed response model can be further generalized by integrating attributes of additional modalities (such as PET- positron emission tomography) into the process. Increased reliability in lateralizing TLE cases using the proposed response model reinforces the notion that ECoG in a number of cases may be circumvented.
Introduction: Temporal lobe epilepsy (TLE) is the most widespread type of epilepsy with the most successful resection outcome [1]. Interhemispheric variations detected in the images of T1-weighted and fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI), and ictal and interictal single photon emission computed tomography (SPECT), and in the indices of mean diffusivity (MD) and fractional anisotropy (FA) of diffusion tensor imaging (DTI), are within the established markers of TLE laterality [2-7]. However, current non-quantitative imaging evaluations may not optimally incorporate the imaging information into the decision-making process prior to resection of mesial temporal structures. We hypothesize that quantitative TLE lateralization response models of MRI, DTI, and SPECT neuroimaging attributes will optimize the selection of surgical candidates and reduce, in some cases, the need for extraoperative electrocorticography (ECoG).
Methods:
Patients and Treatment. We included 138 TLE patients (60 males,78 females) who underwent resection of temporal structures with Engel I outcomes (i.e., seizure-free for 2 years postoperatively) who had at least one of T1-weighted MRI, FLAIR, DTI or SPECT (ictal and interictal) imaging data accessible. The left or right temporal lobe was epileptogenic in 67 and 71 patients, respectively. Extraoperative ECoG was required in 49 of these patients.
Preoperative Data Acquisition. MRI of the patients was done on a 1.5T or a 3.0T MRI system (Signa, GE, Milwaukee, USA) and included inversion recovery spoiled gradient echo (IRSPGR) T1-weighted and T2-weighted FLAIR images. Thirty-six of the patients underwent DTI using echo planar imaging (EPI) on the 3.0T MRI system with b-value = 1000 and number of diffusion gradient directions = 25. For ictal SPECT imaging, 42 of the patients were injected 99mTc- ethylene cysteine dimer (ECD) (99mTc-ECD) at a dose of 550 MBq within 56 sec of seizure activity. Within 2-3 hours post injection of radiotracer, the patients underwent a triple-head Picker gamma camera 3000XP imaging system (Picker International, Inc., Cleveland Heights, OH). When there was no seizure activity recorded for a time window of at least 24 h, interictal SPECT imaging was performed for the same set of patients. Twenty-five nonepileptic control subjects were also included in this study, who underwent the same 3.0T MRI system with the same T1-weighted, FLAIR, and DTI parameters mentioned above and twenty of them also underwent SPECT imaging.
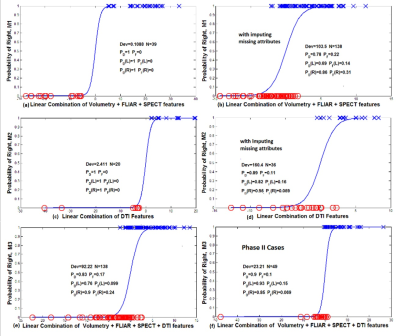
Lateralization Response Models. The multivariate features were extracted [3,4,7,8] and integrated to develop multimodal response-driven models (M1 to M3, see definitions in Fig. 1) [9] to lateralize individual TLE patients. Leave-one-out cross-validation was performed to evaluate generalizability of the logistic function to an independent data set and its accuracy in practice [10]. The fit deviance (Dev), as a generalized residual sum of squares, was used to evaluate the goodness-of-fit for the response models. The performance of the response models was assessed by comparing the probability of detection and false alarm) of the epileptogenic side.
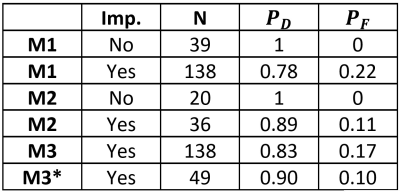
Results: M1 achieved the probability of detection of 1 with no false alarm for the epileptogenic sides in 39 TLE patients who had T1, FLAIR and SPECT imaging (Fig. 1-a). However, for all TLE cases that had at least one of these images and imputing the mean value of the measured attributes of the control cases into the corresponding missing attributes, the probabilities of detection and false alarm by M1 were 0.78 and 0.22, respectively (Fig. 1-b). Similarly, the full DTI model M2 achieved the probability of detection of 1.0 with no false alarm in 20 TLE patients who had all DTI features available (Fig. 1-c). For 36 TLE cases that had at least one DTI feature and imputing the mean value of the measured attributes of the control cases into the corresponding missing attributes, the probabilities of detection and false alarm by M2 were 0.89 and 0.11, respectively (Fig. 1-d). Finally, by incorporating all multivariate attributes for 138 TLE cases that had at least one imaging attribute and imputing the mean value of the measured attributes of the control cases into the corresponding missing attributes, the all-inclusive model M3 reached the normalized fit deviance of 0.74±0.02, the probability of detection of the epileptogenic side of 0.83, and of the false alarm probability of 0.17 (Fig. 1-e). By lateralizing TLE using the multivariate response model M3, the epileptogenic side was detected for 90% of patients (93% of left and 85% of right TLE), who underwent ECoG (Fig. 1-f, Table 1).
Conclusion: Increased reliability in lateralizing TLE cases using the proposed multivariate neuroimaging response model reinforces the notion that ECoG in a number of cases may be circumvented.
Acknowledgements
Research was supported in part by NIH grant R01-EB013227.References
[1] Engel Jr J. New England Journal of Medicine, 1996. [2] Akhondi-Asl A. et al. Neuroimage, 2011. [3] Jafari-Khouzani K. et al. Neuroimage, 2010. [4] Jafari-Khouzani K. et al. Epilepsy Research, 2011. [5] Nazem-Zadeh M.-R. et al. NeuroImage:Clinical, 2016. [6] Nazem-Zadeh M.-R. et al. Journal of the neurological sciences 2014. [7] Nazem-Zadeh M. R. et al. Medical physics, 2012. [8] Jenkinson M. et al. Medical image analysis, 2001. [9] Hosmer D. W. et al. Applied logistic regression. Wiley, 2013. [10] Picard R. R. et al. Journal of the American Statistical Association, 1984.Figures