Stefano Trebeschi1, Joost J. M. van Griethuysen1, Doenja M. J. Lambregts1, Max J Lahaye1, Frans C. H. Bakers2, Roy F.A. Vliegen3, Emile Voest4, Regina G.H. Beets-Tan1, and Hugo J.W.L. Aerts5
1Radiology, Netherlands Cancer Institute, Amsterdam, Netherlands, 2Radiology, Maastricht University Medical Center, Maastricht, Netherlands, 3Radiology, Zuyderland Medical Center Heerlen, Heerlen, Netherlands, 4Medical Oncology, Netherlands Cancer Institute, Amsterdam, Netherlands, 5Radiation Oncology and Radiology, Dana Farber Cancer Institute, Boston, MA, United States
Synopsis
Aim of this investigation was to assess the predictive value of MR Radiomics as predictive biomarker for locally advanced rectal carcinoma. Through univariate analysis and unsupervised biclustering we found significant associations between diffusion radiomic textures and complete response in a multi-center cohort. The results suggest the viability of Radiomics as biomarker and puts emphasis on image quality.
Introduction
Pre-treatment prediction of response to neoadjuvant chemoradiotherapy is a relevant issue in rectal cancer as it may allow to further optimize neoadjuvant treatments aiming to increase response rates. Radiomics refers to a collection of analytical methods to convert images into high dimensional data via a set of quantitative morphological descriptors called“features”. Recurrent patterns among features of responders versus non-responders can be discovered via Machine Learning (ML) techniques tailored to analyze large datasets. Aim of this study is to assess the value of Radiomics using multiparametric MRI (mpMRI) for pre-treatment response prediction in locally advanced rectal cancer (LARC).Materials and Methods
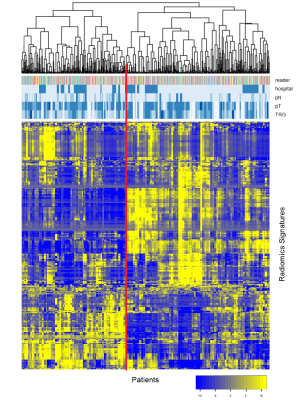
We retrospectively assessed the primary staging mpMRIs of 142 LARC-patients (from 2 institutions, n=91 and n=49) performed before onset of chemoradiotherapy (CRT). The mpMRI-protocol included T2 weighted and diffusion-weighted imaging (DWI; B0 and B1000/1100) sequences and ADC maps derived from the DWI-sequence. Tumors were semi-automatically segmented on B1000/1100 DWI by a region-growing algorithm and then manually adapted by 4 readers (2 expert; 2 non-expert), yielding 5 segmentations (volumes of interest; VOI) in total. MR volumes were standardized to zero mean and one standard deviation. 3758 Radiomic features were extracted from the tumor VOIs. Feature stability was assessed using the intraclass correlation coefficient (ICC) across different readers/segmentations. Predictive association between complete tumor response (ypT0) and residual tumor (ypT1-4) after CRT was assessed by ROC-curve analysis with False Detection Rate (FDR) 20% and unsupervised biclustering analysis. Standard of reference was histopathology after surgery (or long term follow-up after watchful waiting).Results
Out of 3758 initial Radiomic features, 1691 were stable (ICC ≥ 0.75) across different readers, of which ± 300 features per reader showed significant performance after FDR correction. However, no features extracted from the semi-automatic segmentation survived FDR correction. A final subset of 220 features remained stable and performant across all readers. These features resulted in a mean AUC of 0.67 (range 0.64-0.74) to predict a complete response and a mean ICC of 0.81 (range 0.75-0.95). Discussion
Our analysis revealed a predictive association between pre-treatment mpMRI radiomic features, in particular DWI texture features, and complete response in patients with rectal cancer undergoing neo-adjuvant CRT:220 robust features were identified with a mean predictive performance of AUC 0.67 to predict a complete response before onset of treatment. Semi-automated segmentations were not useful, indicating that manual segmentation input is required. Interestingly, delineations by both experienced and inexperienced readers showed comparable results, suggesting that the selected features are robust and do not necessarily require highly expert input. Although the bi-institutional nature of the study did not seem to pose a problem, our results put emphasis on image quality: unlike volumetric measurements, textural features – which measure the interaction of neighbouring voxels within a region of interest – could potentially suffer from MR artefact and poor spatial resolution. Radiomics analysis of pre-treatment MRI may contribute to the pre-treatment prediction of response to treatment in patients with rectal cancer. This is of clinical importance to further optimize neoadjuvant treatment; (i.e. avoid potentially harmful CRT in patients unlikely to respond, while intensifying treatment (by radiation dose escalation) in patients susceptible to treatment, thereby enhancing response rates. Ultimate aim is to increase the number of complete responders as these patients may be candidates for organ-preserving treatments (‘wait-and-see’) instead of surgical resection. Conclusions
With this study, we demonstrated that Radiomics features derived from multiparametric MRI seem to hold potential as pre-treatment predictive biomarkers. As a follow-up step we aim to build a computer-aided predictive model and validate our results in larger independent, mutlicenter patient cohorts. Acknowledgements
No acknowledgement found.References
[1] Aerts, Hugo JWL, Emmanuel Rios Velazquez, Ralph TH Leijenaar, Chintan Parmar, Patrick Grossmann, Sara Cavalho, Johan Bussink et al. "Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach." Nature communications 5 (2014).