2745
Classification of Different Episodic Memory Tasks by Time Points using a Deep Neural Network1Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, United States, 2University of Colorado, Boulder, CO, United States
Synopsis
Classification of different episodic memory tasks by time points is challenging because the signal-to-noise ratio in affected brain regions of the medial temporal lobes is low and similar brain regions (such as the hippocampus) contribute to memory activation. No studies have implemented a deep neural network (DNN) to classify memory tasks at each fMRI time point using whole-brain data. We have implemented a region-of-interest based DNN framework and applied it to classify three different episodic memory tasks. Results indicate that this DNN classifier can accurately discriminate between all these tasks.
Introduction
Deep neural networks (DNN) were recently applied in neuroimaging research for tissue segmentation, group classification, task classification, and prediction of the disease severity of patients [1,2,3,4]. No studies have implemented DNN to classify different memory tasks by time points using whole-brain data. Classifying memory tasks by time point is challenging, because some brain regions, such as the hippocampus, may be involved in all episodic memory tasks. We have constructed a region-of-interest (ROI) based DNN framework and applied it to classifying three different episodic memory tasks.Methods
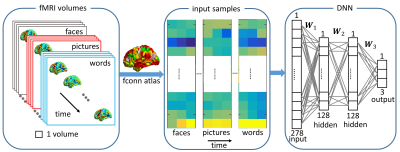
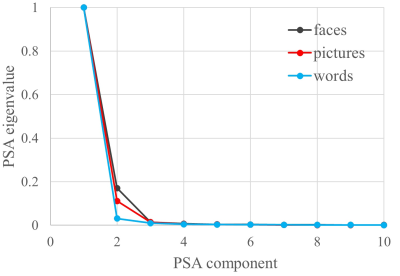
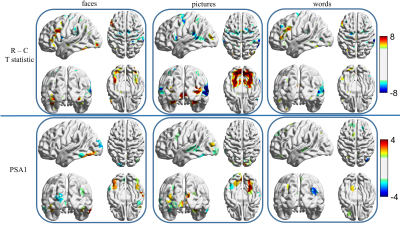
Subjects: Episodic memory task fMRI data of 16 subjects were acquired on a 3.0T GE MRI scanner. All subjects were scanned with three memory tasks consisting of faces paired with occupations, natural scenery pictures, and word pairs describing common objects. Each memory task consisted of six periods of encoding (21sec), distraction (11sec), recognition (42sec), and instruction (5sec). Analysis: Only the recognition blocks were extracted for task classification. A schematic diagram of task classification is shown in Fig.1. Each time point in the recognition blocks is treated as a sample, and the mean BOLD signal intensities in ROIs, determined by the fconn atlas [5], are the input features to the DNN classifier. The DNN is constructed with an input layer, two 128-node hidden layers, and an output layer with 3 nodes corresponding to the three memory tasks. The commonly used ReLU [6] activation function was utilized in the DNN framework. The search direction of weight matrices ($$$W_1$$$, $$$W_2$$$ and $$$W_3$$$) is updated with an adaptive gradient descent method [7] and the dropout technique [8] is applied to alleviate overfitting. The softmax function is used for the output layer so that the output can be interpreted as the posterior probability of each corresponding task. We trained the DNN with a leave-one-subject-out cross validation method and predicted the tasks with the left-out subject. To determine the importance of each ROI in classifying different tasks, we applied a principal sensitivity analysis (PSA) [9] to the trained DNN classifier. Similar to principal component analysis maximizing the variance along a set of orthogonal directions in feature space, PSA searches the orthogonal directions in the ROI-feature space and ranks these directions in order of eigenvalues. The direction with maximal eigenvalue is the most important one to discriminate one task from all the other tasks. To further validate the maps found by PSA, we compared the maps obtained with the group activation map for each task for contrast “recognition-control” (R-C).Results
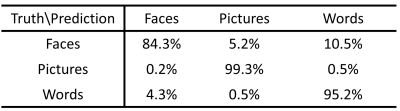
The DNN classifier achieves 94.7% accuracy in predicting the different memory tasks at individual fMRI time points. Table 1 shows the accuracy for each specific task. The time points for the picture task can be predicted with near perfection, and accuracy for the face task is the lowest. Fig.2 shows the top ten eigenvalues computed from PSA for all three tasks, with the largest eigenvalue normalized to be one for the purpose of visualization. The first component found by PSA is most important since the first eigenvalue dominates all others. In Fig.3, the top panel shows the group activation maps for these three tasks. The bottom panel shows the direction obtained from PSA with maximal eigenvalue. The activations in the fusiform gyrus are distinct among these three tasks. The activated fusiform area for the picture task is located medially in respect to the activated area for the face task. In contrast, there is little activation in the fusiform gyrus for the word task. The regions found in the PSA maps are consistent with the group maps.Discussion and Conclusion
We constructed a deep learning framework by stacking multiple dense layers to extract non-linear features from episodic memory data. The DNN is highly accurate in classifying different memory tasks by time point, however, it misclassifies the face task as the word task with a 10.5% rate. Both face and word tasks show activation in language regions, such as left inferior frontal gyrus and left inferior temporal gyrus. Since the face task had a verbal label (occupation), it likely explains the difficulty in discriminating face and word tasks. This DNN framework is also robust against the heterogeneity among subjects since testing was performed on independent subject data.Acknowledgements
The study is supported by the National Institutes of Health (grant number 1R01EB014284 and P20GM109025).References
[1] Wang et al., 2015. Deep convolutional neural networks for multi-modality isointense infant brain image segmentation. NeuroImage, 108, pp214-224.
[2] Suk et al., 2014. Hierarchical feature representation and multimodal fusion with deep learning for AD/MCI diagnosis. NeuroImage, 101(1), pp 569-582.
[3] Jang et al., 2017. Task-specific feature extraction and classification of fMRI volumes using a deep neural network initialized with a deep belief network: Evaluation using sensorimotor tasks. NeuroImage, 145. Pp 314-328.
[4] Cole et al., 2017. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage, 163, pp 115-124.
[5] Shen et al., 2013, Groupwise whole-brain parcellation from resting-state fMRI data for network node identification.
[6] Jarrett, K., Kavukcuoglu, K., Ranzato, M., LeCun, Y., 2009. What is the best multi-stage architecture for object recognition? In: 2009 IEEE 12th International Conference on Computer Vision. IEEE, pp. 2146-2153.
[7] Zeiler, Mathew D., 2012, ADADELTA: an adaptive learning rate method
[8] Hinton, G. E., Srivastava, N., Krizhevsky, A., Sutskever, I., Salakhutdinov, R. R., jul 2012. Improving neural networks by preventing co-adaptation offeature detectors. arXiv preprint arXiv:1207.0580, 1-18.
[9] Koyamada, S., Koyama, M., Nakae, K., Ishii, S., dec 2014. Principal Sensitivity Analysis. arXiv preprint arXiv:1412.6785, 1-13.
Figures