2742
Prognostic-value of imaging markers for the prediction of the clinical evolution in Alzheimer’s disease1Qynapse, Paris, France, 2École polytechnique fédérale de Lausanne, Paris, France
Synopsis
Predicting the individual clinical course remains a major issue in biomarker research in Alzheimer’s disease to adapt the therapeutic care of patients. Imaging data may contain valuable early markers of the clinical evolution of AD. In this study, we investigated the prognostic value of some imaging markers for the prediction of the clinical evolution of mild cognitive impairment (MCI) and AD patients over 24 months through both the conversion and the cognitive decline problems. With a rigorous validation scheme, for each clinical outcome, we built competitive predictive models on the ADNI cohort which are highly generalizable to other independent cohorts (OASIS and AddNeuroMed).
INTRODUCTION
Alzheimer’s disease (AD) is the most prevalent form of dementia characterized by pathological brain atrophies that appear even before the first objective amnestic impairments1,2. Several studies demonstrated the strong correlation between structural features extracted from MRI scans and the disease progression, making them as valuable markers of prognostic3,4. However, the diagnosis may be complex because the transition from mild cognitive impairment to dementia is highly variable from one patient to another. Moreover, brain changes at the early stage of the disease are subtle making it challenging to automatically detect the imaging pattern that, combined with clinical and demographic data, has the highest early-prognostic value5,6,7. Predictive models of Alzheimer’s disease evolution have to be accurate and robust for decision-support use in clinical routine considering differences between imaging- and clinical- protocols8 9,10. Unfortunately, most of the studies used one cohort to both train and validate their models missing to prove their generalizability and robustness to between-cohort variability. In this study, we focused on the research and development of accurate prognosis tools to a) identify patients with a mild cognitive impairment (MCI) likely to convert to AD and b) quantify the cognitive decline by predicting MMSE clinical score. We pursued two main objectives: 1) build robust predictive models to predict the conversion and cognitive decline within 24 months after baseline based on demographic, imaging and clinical features acquired at baseline; 2) assess the added value of our imaging markers.DATA
As suggested by comparative studies and challenges11, the use of publicly available datasets enable direct performance comparison between studies on the same dataset. Therefore, for comparison and generalization purposes, we used data from three public databases to achieve our medical objectives: ADNI12, OASIS13 and AddNeuroMed14 databases. These three datasets have different MR-protocols, as well as different demographic and clinical characteristics. From MRI scans, nine markers were automatically extracted with the QyScore software15. These markers are the normalized volumes of some cerebral Regions Of Interest (ROI): hippocampus (left, right hemisphere and total), the amygdala (left, right and total), the grey matter, the white matter, and the total intracranial volume (TIV). As additional input data in our predictive models, we integrated demographic, genetic (ApoE4) and cognitive data (MMSE score) recorded at baseline.METHODS
We performed a benchmark by comparing various off-the-shelf supervised multivariate methods (linear- and radial- basis support vector machines (SVM), L1- and L2- regularized logistic regression (RLR) and random forest), on several combinations of data source (Imaging, demographic and clinical). We implemented a rigorous analysis algorithm composed of a 2-level validation:
- Models’ optimization and validation on ADNI with an inner and outer cross-validation to identify the optimum models (i.e. the couples composed of the best classifier and input set) for each clinical outcome
- Training of the optimum models on ADNI and validation on both OASIS and AddNeuroMed datasets to assess their generalizability power for clinical use.
Each source of data was independently tested and then combined to assess the predictive value of imaging markers for the prediction of the conversion and MMSE score. Performance scores were computed with the Area Under ROC Curve (AUC) for the conversion problem and with the Pearson’s correlation coefficient (r) for the prediction of the MMSE score. Additionally, the statistical significance (i.e. p-value) of these performance scores were estimated with a 1000-permutation test.
RESULTS
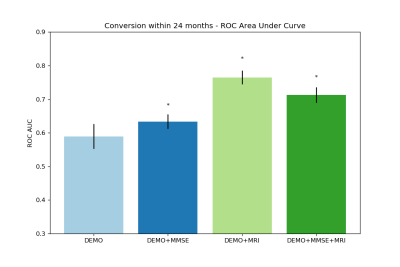
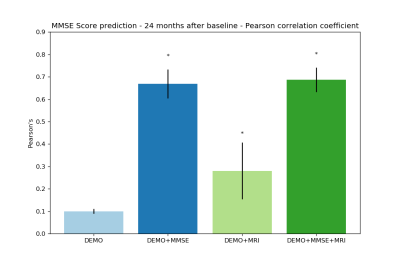
To predict the conversion within 24 months after baseline, the best model, using a L1-regularized logistic regression on baseline imaging and demographic features, achieved 76% of AUC on ADNI (see Figure 1). The same statistically significant predictive power was achieved in generalization on both AddNeuroMed and OASIS. In comparison, if we considered clinical and demographic features alone at baseline, our predictive models achieved at best 63% of AUC. For the MMSE prediction at 24 months, Lasso model applied to baseline imaging, demographic and MMSE features got a Pearson’s correlation coefficient of 70% on ADNI and 88% on both AddNeuroMed and OASIS (see Figure 2). In comparison to the models based on clinical and demographic features, adding imaging markers led to slightly higher and more stable performance.DISCUSSION
For the conversion problem, based on demographic and a few imaging markers, we obtained comparable results to the best reported on similar studies16. For the prediction of the MMSE score, to our knowledge, we outperformed the performances obtained by similar studies17. A major result of our study is that we successfully demonstrated the generalizability of our models on two independent datasets, OASIS and AddNeuroMed, after their training on the whole ADNI database. It is also worth noting that we proved the predictive value of the imaging markers in comparison to the MMSE clinical feature commonly used to make the diagnosis.Acknowledgements
No acknowledgement found.References
1 S. Rathore, M. Habes, M.A. Iftikhar, A. Shacklett, C. Davatzikos, A review on neuroimaging-based classification studies and associated feature extraction methods for Alzheimer's disease and its prodromal stages. Neuroimage vol. 155, Pages 530-548, July 2017.
2 M.F. Folstein, S.E. Folstein, and P.R. McHugh, “mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research, vol. 12, no. 3, pp. 189-198, 1975.
3 R.S. Desikan, H.J. Cabral, C.P. Hess, W.P. Dillon, C.M. Glastonbury, M.W. Weiner, N.J. Schmansky, D.N. Greve, D.H. Salat, R.L. Buckner, et al., Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain, vol. 132, no. 8, pp. 2048-2057, 2009
4 G.B. Frisoni, N.C. Fox, C.R. Jr Jack, P. Scheltens, P.M. Thompson, The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol, 6(2):67-77, Feb 2010.
5 I.O. Korolev, L.L. Symonds, A.C. Bozoki, Predicting progression from mild cognitive impairment to Alzheimer's dementia using clinical, MRI, and plasma biomarkers via probabilistic pattern classification. PLoS One 11, 2016.
6 R. Casanova, F.C. Hsu, K.M. Sink, S.R. Rapp, J.D. Williamson, S.M. Resnick, M.A. Espeland, Alzheimer's disease risk assessment using large-scale machine learning methods. PLoS One 8, 2013.
7 C. Misra, Y. Fan, C. Davatzikos, Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: results from ADNI. Neuroimage 44, 1415–1422, 2009.
8 A.V. Lebedev, E. Westman, G.J. Van Westen, M.G. Kramberger, A. Lundervold, D. Aarsland, H. Soininen, I. Kloszewska, P. Mecocci, M. Tsolaki, B. Vellas, S. Lovestone, A. Simmons, Random Forest ensembles for detection and prediction of Alzheimer's disease with a good between-cohort robustness. Neuroimage: clinical Volume 6, Pages 115-125, 2014.
9 G. Spulber, A. Simmons, J-S. Muehlboeck, P. Mecocci, B. Vellas, M. Tsolaki, I. Kloszewska, H. Soininen, C. Spenger, S. Lovestone, L-O. Wahlund, E. Westman, An MRI-based index to measure the severity of Alzheimer's disease-like structural pattern in subjects with mild cognitive impairment, J Intern Med., 273(4): 396–409, Apr. 2013.
10 E. Westman, A. Simmons, J.S. Muehlboeck, P. Mecocci, B. Vellas, M. Tsolaki, I. Kloszewska, H. Soininen, M.W. Weiner, S. Lovestone, C. Spenger, L.O. Wahlund, AddNeuroMed consortium; Alzheimer's Disease Neuroimaging Initiative. AddNeuroMed and ADNI: Similar patterns of Alzheimer's atrophy and automated MRI classification accuracy in Europe and North America, Neuroimage, 1;58(3):818-28, Oct. 2011.
11 E.E. Bron, M. Smits, W.M. van der Flier, H. Vrenken, F. Barkhof, P. Scheltens, J.M. Papma, R.M. Steketee, C. Mendez Orellana, R. Meijboom, et al., Standardized evaluation of algorithms for computer-aided diagnosis of dementia based on structural MRI: the CADDementia challenge. Neuroimage 111, 562–579, 2015.
12 C.R. Jack Jr, M.A. Bernstein, N.C. Fox, P. Thompson, G. Alexander, D. Harvey, B. Borowski, P.J. Britson, J.L. Whitwell, C. Ward, et al., The alzheimer's disease neuroimaging initiative (adni): MRI methods," Journal of magnetic resonance imaging: JMRI, vol. 27, no. 4, p. 685, 2008.
13 D.S. Marcus, A.F. Fotenos, J.G. Csernansky, J.C. Morris, and R.L. Buckner, Open access series of imaging studies: longitudinal mri data in nondemented and demented older adults, Journal of cognitive neuroscience, vol. 22, no. 12, pp. 2677-2684, 2010.
14 S. Lovestone, P. Francis, I. Kloszewska, P. Mecocci, A. Simmons, H. Soininen, C. Spenger, M. Tsolaki, and B. Vellas, Addneuromed|the european collaboration for the discovery of novel biomarkers for alzheimer's disease, 2009.
15 http://www.qynapse.com/index_fr.html#technology
16 F. Falahati, E. Westman, and A. Simmons, Multivariate data analysis and machine learning in Alzheimer's disease with a focus on structural magnetic resonance imaging. Journal of Alzheimer's disease: JAD, vol.41, issue.3, pp.685-708, 2014.
17 S. Duchesne, A. Caroli, C. Geroldi, D.L. Collins, G.B. Frisoni, Relating one-year cognitive change in mild cognitive impairment to baseline MRI features. Neuroimage, 1; 47(4):1363-70, Oct 2009.
Figures