2741
Is it possible to estimate recanalization effect for acute ischemic stroke patients using a single deep learning model?1Center of Functionally Integrative Neuroscience and MINDLab, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark, 2Cercare Medical, Aarhus, Denmark
Synopsis
Every year, 13 million people suffer acute ischemic stroke. Brain tissue infarcts permanently within hours after stroke onset and rapid recanalization is therefore of utmost importance. In this project, we aim to estimate recanalization effect by a single convolutional neural network customized to include magnetic resonance imaging biomarkers as well as individual recanalization information. This is in contrast to the traditional approach which is splitting the data set according to the recanalization information and training several models. We find a significant recanalization effect and believe this to be an important step towards an automated decision support system.
Introduction:
Every year, 13 million people suffer acute ischemic stroke. Brain tissue infarcts rapidly (two million neurons die every minute a major artery is occluded1) and rapid recanalization is therefore of utmost importance to ensure optimal outcome. Current magnetic resonance imaging allows assessment of irreversibly damaged tissue and surrounding tissue suffering reduced blood delivery. Salvageable tissue (or the ischemic penumbra) is defined as tissue with reduced blood supply, which has not already infarcted, and may be saved by quick recanalization. In this project, we aim to estimate the effect of such recanalization. The traditional approach would be to split the data according to individual recanalization information and train several predictive models. However, we do not think this is an optimal approach for data demanding methods such as convolutional neural networks (CNN). Therefore, we aim to estimate recanalization effect using a single model customized to include imaging biomarkers and individual recanalization information and capable of automatically estimating the recanalization effect.Methods:
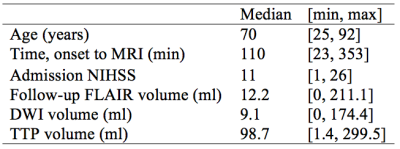
133 acute ischemic stroke patients from the I-KNOW multicenter2 (53) and perconditioning3 (80) studies were randomly divided into training (112) and test (21) sets. 96 patients recanalized (defined as a TIMI score of 2 or 3) and 37 did not (TIMI score of 0 or 1). The acute MRI protocol included diffusion-weighted imaging (DWI), T2 fluid-attenuated inversion recovery (T2-FLAIR) imaging, and gradient-echo (GE) dynamic susceptibility contrast perfusion-weighted imaging (DSC-PWI) with echo time 30-50 ms and repetition time 1500 ms. Gadolinium contrast dose 0.1 mmol/kg at injection rate 5mL/second was followed by a saline chaser. Figure 1 shows patient characteristics and further details are listed in Hansen et al4. We modified a deep CNN5 consisting of 37 layers (presented at ISMRM 20176) to take both biomarkers and recanalization information as input. The predictions were based on T2-FLAIR, trace DWI, apparent diffusion coefficient (ADC) and the following perfusion biomarkers: mean transit time (MTT), cerebral blood volume (CBV), cerebral blood flow (CBF), cerebral metabolism of oxygen7 (CMRO2), relative transit time heterogeneity (RTH), delay and recanalization information. The resulting model’s ability to estimate recanalization effect was evaluated on the independent test set using the predicted final infarct volume compared to the follow-up lesion volume. A Wilcoxon signed-rank test was used to test for a significant difference between predicted final infarct volume ±recanalization. Volumes are indicated in ml as (mean±std.dev).Results:
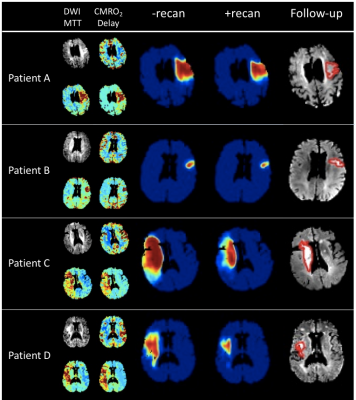
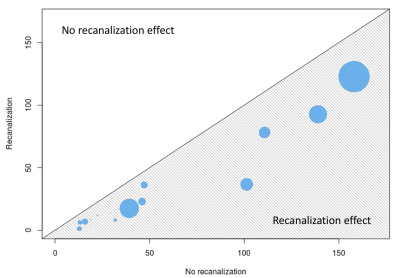
Figure 2 shows examples from the predictive model. For patient A and B, the mismatch between the diffusion and perfusion weighted imaging lesions are small (12 and 3 ml). For patient C and D, the mismatch is larger (108 and 130 ml). The patients with a large mismatch have a substantial recanalization effect, whereas patients with a small mismatch show almost no recanalization effect. This is consistent with the well-esteemed penumbra model. The highest predicted final infarct volume was obtained for -recanalization (41.9±45.8) with a significant difference (p<0.0001) to +recanalization (24.2±33.4). The difference is also apparent in Figure 3 showing predicted final infarct volume ±recanalization. Patients with a small follow-up lesion volume (a small dot in Figure 3) also have a small predicted final infarct volume for both methods. The difference between ±recanalization is more apparent for larger follow-up infarct volumes.Discussion:
We showed a significant difference in final infarct volume between the model’s prediction with and without recanalization, with no recanalization leading to larger final infarct volumes. This corresponds to the penumbra model. The use of a single model capable of including recanalization information is a better use of data compared to training several models. Using this method, it is possible to include other types of information influencing the stroke outcome and thereby make even more individualized and improved predictions. We decided to assess the ability of the CNN to identify recanalization differences based. The same methodology could equally well be used to examine the direct effects of treatments.Conclusion:
The recanalization effect was significant, with –recanalization yielding a higher volume of final infarct. The new model paradigm has shown recanalization effect estimation and thereby a potential of clinical information in automated decision support systems providing recommendations for personalized treatment and thereby hopefully better outcome for the individual patient.Acknowledgements
No acknowledgement found.References
1. Saver JL. Time is brain - quantified. Stroke. 2006;37:263-266
2. I-KNOW. Integrating information from molecules to man: Knowledge discovery accelerates drug development and personalized treatment in acute stroke. 2006
3. Hougaard KD, Hjort N, Zeidler D, Sorensen L, Norgaard A, Thomsen RB, et al. Remote ischemic perconditioning in thrombolysed stroke patients: Randomized study of activating endogenous neuroprotection - design and mri measurements. Int J Stroke. 2013;8:141-146
4. Hansen MB, Nagenthiraja K, Ribe LR, Dupont KH, Ostergaard L, Mouridsen K. Automated estimation of salvageable tissue: Comparison with expert readers. J Magn Reson Imaging. 2016;43:220-228
5. Badrinarayanan V, Kendall A, Cipolla R. Segnet: A deep convolutional encoder-decoder architecture for image segmentation. ArXiv e-prints. 2015;1511
6. Nielsen A, Hansen MB, Mouridsen K, Boldsen JK. Deep learning: Utilizing the potential in data bases to predict individual outcome in acute stroke. ISMRM. 2017
7. Jespersen SN, Østergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32:264-277
Figures