2740
Deep Recurrent Neural Network Based Learning for Determining Structural Changes in Brain MRE: Towards Early Detection of Alzheimer’s1MRI, GE Healthcare, Bangalore, India
Synopsis
Alzheimer’s Disease (AD) is a type of dementia which is now known to be the leading cause of death in the United States. Hence, early detection of AD is crucial for treatment planning and preventive measures before patient develops irreversible brain trauma. Deep learning (DL) is a robust machine learning technique used for classification to extract low-to high-level features. Previous studies have used DL to classify functional MRI data of Alzheimers subjects. However, none have employed DL to classify the ealsticity changes in brain MRE data. As a first step towards early diagnosis of AD we have developed a deep recurrent neural learning scheme to classify structural and elasticity changes in brain MRE.
Intoduction
Worldwide around 2 million people suffer from neuro-degenerative disorders of which Alzheimer’s disease (AD) is the most predominant. However, there is no gold standard for clinicians to decide the type of treatment that should be provided to patients irrespective of patient reports initial symptoms of AD or has prolonged Alzheimer’s [1]. Therefore, there is a pressing need for the development robust classification, and predictive modeling schemes. Several studies in past have analyzed the Alzheimer’s with fMRI scans and have tried to classify the findings. But these studies did not investigate structural changes in brain which occur due to variations in elasticity and stiffness of the ageing brain [2]. In our study, we propose a novel deep learning, recurrent neural network for analyzing the elasticity and stiffness changes in the brain using the magnetic resonance elastography technique. The method employed in our study is robust and is embedded for multi-level convolution neural network which is used as input to recurrent neural network which classifies structural change based on information of elasticity and stiffness changes. The proposed approach exceeds significantly previous results obtained on cross-patient classifiers both in terms of sensitivity and false positive rate. Furthermore, our model proves to be robust to stiffness changes and variable structural deformation due to ageing.Methods
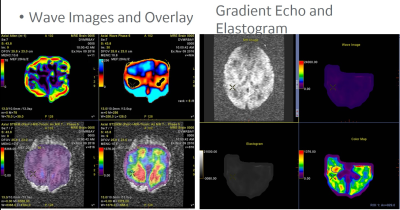
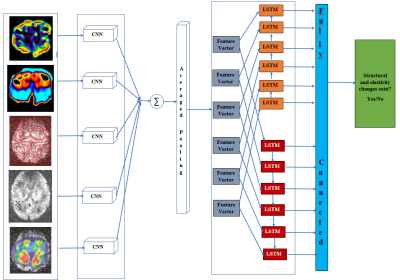
We performed MRE of brain with shear wave frequencies in the range of 5 -20 Hz. For every variation in frequency the corresponding stiffness and elasticity maps were recorded. A data repository was generated to stored the gradient echo, stiffness, and elasticity map. A perfusion based multi voxel technique was employed by adjusting the cost function of the balanced rectangular gradients . Level set segmentation approach was used to analyze the localized structural changes. This post processed MRE brain data was treated as an input to the learning network (Figure.1). In our work, we have established a recurrent convolution architecture designed to capture spectral, temporal, and spatial patterns from the variations that are found in elasticity and stiffness of various brain regions. This data represent the structural change patterns (Figure.1). Further, the data set obtained is combined with an image gradient based representation of Alzheimer’s incorporating the elasticity and stiffness knowledge, the model learns a patient-independent representation of structural change patterns. First, the elasticity map is projected into an image representation , which is then fed Second, a recurrent convolution neural network is trained to predict whether the corresponding image contains a elastic and structural change. Further, Recurrent neural networks, illustrated in Figure 2, are a special type of artificial neural network that contain loops in the neural architecture to allow for information to persist over time. More specially, we use Long short Term Memory units (LSTM), which have proven elective to disuse information over time sequences. This is particularly relevant to analyze elasticity data, given that early symptoms of Alzheimer’s. Figure 2 illustrates the pipeline of our detection architecture. To connect the convolution network with the Recurrent network, we feed the output of the network into one of the feature vector blocks of the architecture in Figure 2. The recurrent architecture takes as input 60 such blocks.Results
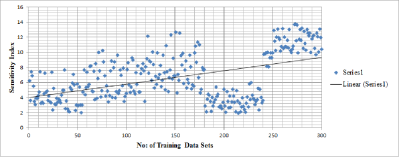
Our results show that the actual sensitivity drops significantly on many of the patients compare to patient specific to certain Alzheimer’s symptoms (Figure .3). The main advantage of our deep architecture lies in its ability to categorize non structural and structural changes based on the elasticity and stiffness variations captured at various brain regions. We can observe that the sensitivity index increased with large data sets and decreases with small MRE data sets. Further, based on the sensitivity data, our deep learning scheme successful captures the stiffness and elasticity changes in the specific regions of brain .Discussion and Conclusions
Traditional deep learning methods typically do not perform accurately on new patients, due to their low capacity to generalize well across different structural change patterns. Indeed,Using convolution neural network and recurrent convolution neural Network (RCNN), we successfully classified structural MRI data of Alzheimer's subjects from normal's based on the stiffness and elasticity variations. The accuracy of test data on trained data reached 90.34%. This experiment suggests us the shift and scale invariant feature vectors extracted by CNN followed by RNN deep learning classification is most powerful method to differentiate clinical data from healthy data in Brain MRE. This approach also helps to expand our deep learning technique to predict more complex neuro degenerative disorders at an early stage.Acknowledgements
The author would like to thank GE volunteers for participating in the MRE study protocol. The author also extends thanks to Vivek Vaidya and Suresh Joel of GE Global Research for insightful training on deep learning.References
1. G. McKhann, D. Drachman, et al., "Clinical diagnosis of Alzheimer's disease Report of the NINCDS-ADRDA Work Group∗ under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease," Neurology, Vol. 34, pp. 939-939, 1984.
2. Karen Simonyan, Andrew Zisserman, "Very deep convolutional networks for large-scale image recognition," arXiv preprint arXiv 1409.1556 , 2014.