2732
Accurate Cerebellum segmentation using a 3D Convolutional Neural Network and fully connected CRF1Medical Physics Group, Institute for Diagnostic and Interventional Radiology, Jena University Hospital, Jena, Germany, 2Section of Experimental Neurology, Department of Neurology, Essen University Hospital, Essen, Germany, 3Erwin L. Hahn Institute for Magnetic Resonance Imaging, University Duisburg-Essen, Essen, Germany
Synopsis
Subject-specific information about the cerebellum serves as an important biomarker in the clinical setting, however segmentation of the cerebellum is a challenging task. We demonstrate the feasibility of automatic cerebellum segmentation using a 3D convolutional neural network followed by a fully connected conditional random fields algorithm. The network was trained using 12 preprocessed T1-weighted images and corresponding manually refined ground truth segmentations. The new approach revealed robustness and similar DICE coefficients with respect to the conventional FreeSurfer approach.
Introduction
The cerebellum serves as a major integrative center for the coordination and planning of movement, timing and motor learning. Subject-specific knowledge about the structure of the human cerebellum is thus essential for an understanding of the functional consequences of neurological cerebellar diseases. Segmentation of the cerebellum is a challenging task especially if the subjects suffer from cerebellar atrophy, for instance, due to degenerative cerebellar ataxia. Known automatic segmentation approaches for the cerebellum are SUIT [1], FreeSurfer [2] or cBeast [3], but the accuracy of the segmentation is often limited requiring manual efforts to correct the segmentation. Very recently, segmentation approaches utilizing convolutional neural networks (CNN) have shown promising results for brain segmentation [4]. Therefore, the aim of this contribution is to propose a CNN architecture for accurate, fast and robust cerebellum segmentation and to evaluate its segmentation with respect to the conventional FreeSurfer brain parcellation pipeline and to manually corrected datasets.Methods
Twenty-two healthy subjects underwent 3 Tesla MRI with whole head T1-weighted imaging (MP-RAGE, TE=3.26ms, TR=2530ms, inversion time (TI)=1100ms, FA=7°, BW=200Hz/Px, voxel size=1mm × 1mm × 1mm). The initial segmentation of the cerebellum and brainstem was carried out with the SUIT toolbox 1 and corrected manually afterwards by two independent observers. These manually refined segmentations were considered as ground truth. Spatially slowly-varying signal inhomogeneities in T1-weighted images were corrected using N4 bias field correction [5]. The N4-corrected T1-weighted images and the corresponding refined masks were linearly registered to common standard space allowing for 7 degrees-of-freedom. To reduce computational demands, the 3D volumes of the T1-weighted images and the masks were cropped to a fixed size of 130 x 130 x 100 mm³, which contained the complete cerebellum. Finally, the signal intensities of each T1-weighted images were adjusted to have zero mean and unit variance in a predefined region given by a cerebellum mask in standard space.
For cerebellum segmentation, we set up a single pathway, 3D CNN with 10 hidden layers using the DeepMedic framework [6]. The network was trained with a training scheme utilizing dense training [6] including 12 subjects, and subsequently applied on the remaining 10 subjects. The segmentation results revealed by the trained CNN were further corrected using a pre-trained fully connected conditional random fields (CRF) algorithm [6].
As comparison, the 10 evaluation subjects were separately segmented using the standard FreeSurfer processing pipeline. We used the DICE coefficient to compare the two automatic segmentations with the ground truth.
Results
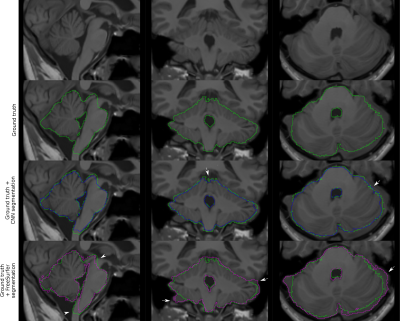
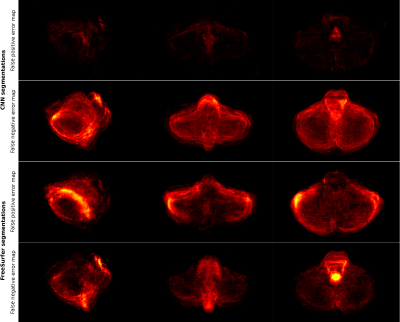
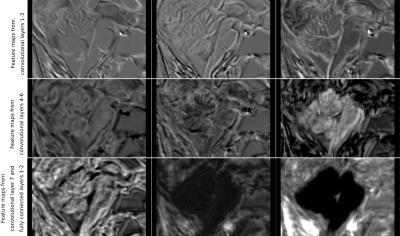
Figure 1 presents the segmentation results for one exemplary subject, including the original dataset, the post-processed CNN segmentation and the FreeSurfer segmentation. Arrows highlight variations between the two automatic segmentations and the ground truth. Average projections of error maps illustrating the distribution of false positives (FP) and false negatives (FN) of the automatic segmentations in relation to ground truth are shown in Figure 2. Figure 3 contains feature maps extracted from the first node of the hidden layers in the CNN through a test session. The average DICE scores were 0.96±0.006 and 0.95±0.008 for the post-processed CNN segmentation and the FreeSurfer segmentation, respectively.Discussion
As a quantitative measure, the calculated DICE score indicates that our CNN and FreeSurfer result in comparative segmentation performances, however, Figure 1 and 2 suggest that several aspects differ. Utilizing FreeSurfer, overestimation appears consistently around the cerebellar posterior lobes and underestimations occur in each end of the brainstem. The underestimation of the brainstem is most likely caused by incongruence in its anatomical definition in FreeSurfer and for manual delineation. In comparison, the post-processed CNN segmentations suffer from general underestimations caused by the CRF post processing step. However, as depicted by arrows in Figure 1, some areas of the CNN segmentation are more confined than the ground truth, hence random errors are eliminated. Both error maps for the post-processed CNN segmentation show evenly distributed FNs and FPs indicating that the CNN framework provides a more robust segmentation approach than FreeSurfer.Conclusion
Using the proposed preprocessing pipeline, 3D CNN and fully connected CRF we demonstrated deep learning to be an efficient method for cerebellum segmentation. The approach was comparable with FreeSurfer segmentation, but indicated improved robustness. We expect that our CNN framework is able to cope with atrophied cerebellums of patients as well if appropriate data is supplied in a previous training step.Acknowledgements
Acknowledgment: This work was supported by the German Research Foundation (DFG, DE2516/1-1, TI239/17-1).References
[1] Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. Oct 15 2006;33(1):127-138.
[2] Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. Jul 1 2006;31(3):968-980.
[3] Güllmar D, Pfaffenrot V, Draganova R, Feng X, Reichenbach JR, Timmann D, Deistung A. cBEaST: Cerebellar Brain Extraction based on Nonlocal Segmentation Technique – A comparison with state-of-the-art methods. ISMRM Annual Meeting 2017,#771 Honolulu, Hawaii
[4] Akkus Z, Galimzianova A, Hoogi A, Rubin D, Erickson BJ. Deep Learning for Brain MRI Segmentation: State of the Art and Future Directions. J Digit Imaging (2017) 30:449–459.
[5] Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010 Jun;29(6):1310-20
[6] Kamnitsas K, Ledig C, Newcombe VFJ, Simpson JP, Kane b AD , Menon DK, Rueckert D, Glocker B. Efficient multi-scale 3D CNN with fully connected CRF for accurate brain lesion segmentation. Medical Image Analysis (2017) 36:1–78.
Figures