2723
Improving the image quality of liver DWI using the convolutional neural network-based selection algorithm1Department of Radiology, University of Yamanashi, Chuo, Japan
Synopsis
Diffusion-weighted imaging (DWI) of the liver using a single-shot EPI sequence suffer from motion artifact caused by cardiac motion. The reconstruction of DWI with multiple numbers of excitation including the corrupted echoes due to systolic cardiac motion results in a severe signal loss in the left lobes, even if other echoes in diastolic phase had no artifact. In this study, we propose a selection algorithm to reject the corrupted echoes using convolutional neural network was proposed. The volunteer studies demonstrated that the proposed method improves the image quality of liver DWI.
INTRODUCTION
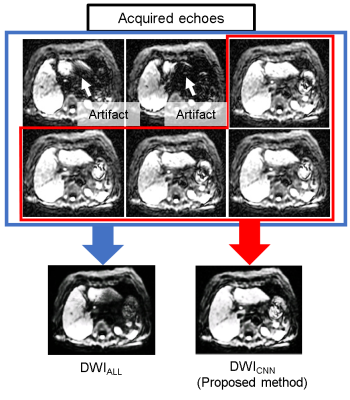
Single-shot EPI-based diffusion-weighted imaging(DWI) in the liver is widely used for the detection and evaluation of the hepatic lesions. In liver DWI, we typically use 4 or 6 times of averaging, i.e. numbers of excitation (NEX) of 4 or 6, to achieve a clinically acceptable signal-to-noise ratio (SNR). Even with the multiple acquisitions, however, liver DWI suffers from the patient’s motion1, especially cardiac motion2-4. An echo (or an acquisition) during diastolic cardiac phase result in acceptable image quality, while an echo during systolic phase makes severe artifacts in the left lobe of the liver. Image reconstruction using the echo with severe artifact due to systolic motion results in unacceptable degradation of DWI, nevertheless rest of the echoes (the other acquisitions) had no artifacts.
In this study, selection algorithm for the liver DWI was developed using the convolutional neural network (CNN) to reject unwanted echoes, e.g. an echo which was affected by systolic motion. The rejection was implemented in the image domain to detect the local artifact. The experimental studies demonstrated that the proposed method improves the quality of liver DWI in clinical patients.
METHODS
We included 40 patients who underwent liver DWI. The sequence was single shot spin-echo echo planar imaging (EPI) with the respiration triggering with NEX of 6 (6 echoes): TR/TE =1000/58ms, FOV=(40cm)2, Matrix=128×192, Slice thickness=4 mm, Slice spacing =1mm, PI factor=2, b = 1000s/mm2. A 3.0 tesla clinical MRI system (Discovery MR750, GE healthcare) with the whole-body coil and 32ch Torso Array were used. The studies were performed with the approval of our institutional review board.
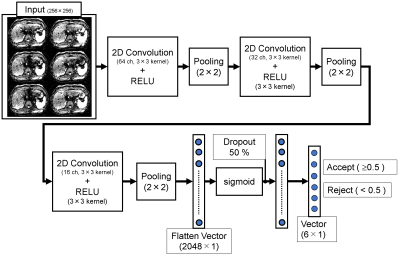
The images from 32 patients were used for training sets (350 slices × 6 echoes), and the rest of the images from the other 8 patients for test sets (127 slices × 6 echoes). First, the acquired six echoes were separately reconstructed. All images in both sets were visually graded by a radiologist using a 5-point scale based on the significance of the artifacts (1=severe artifact affecting >50% of the images; 2= severe artifact; 3=moderate artifact; 4=mild artifact; 5=no artifact). In this study, the images with the score of ≤ 3 were classified as "reject", and 4–5 were "accept". These classified training data (350 slices × 6 echoes) were served for supervised machine learning with CNN. The CNN consists of three two-dimensional convolutions, max-pooling layers, and two fully connected layers (Fig. 1). The parameters of the CNN were trained for 50 epochs with the mini-batch size of 32 sets of images. The algorithm was developed in Python 3.5 with the Keras and Tensorflow.
Using the data from test sets, averaged DWI (127 slices) were reconstructed by two methods; 1) averaging all echoes (DWIALL) and 2) averaging echoes accepted by CNN (DWICNN) as shown in Fig. 2. We compared the classification (accept/reject) by the radiologist and the CNN in all echoes in test sets to calculate a kappa coefficient. The pairwise comparison was performed by a radiologist to see if the image quality was improved by CNN algorithm. The SNRs between DWICNN and DWIALL were also compared.
RESULTS
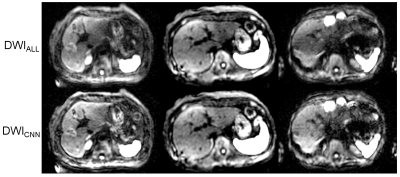
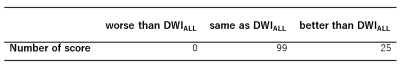
The classification (accept/reject) by the CNN in test sets were well agreed with those by the radiologist (kappa=0.91), that implies the CNN algorithm well replicate the decision of the radiologist. Figure 3 shows the image examples of (top row) DWIALL and (bottom row) DWICNN. In the pairwise comparison of image quality, DWICNN were better than DWIALL in 20 % of the slices, whereas assessed as equivalent in 80% of the slices (Tab. 1). There was no slice which was better in DWIALL. The SNR of DWICNN was significantly lower (P < 0.01) than that of DWIALL. The SNR of DWICNN was decreased by 7.9 % in average.DISCUSSION
Previously, the similar idea (rejection of the data) was implemented in k space domain. In their works, another navigator echo was used for the decision of rejection5-7. Although these rejection approaches improved the image quality, it was difficult to detect local artifacts like the signal loss in the left lobe of the liver. Our approach using machine learning works in the image domain, which enabled to overcome these drawbacks. The decrease of the SNR is a limitation of our study. However, it would be solved by a real-time rejection and reacquisition scheme often used in multi-shot EPI acquisition6,7.CONCLUSION
In this study, we introduced the selection algorithm for DWI using the CNN that works in the image domain to detect the local artifact in the liver. This approach improved the image quality in 20% of the slices in liver DWI.Acknowledgements
No acknowledgement found.References
1. T Taouli, B., et al., (2009). J. Magn. Reson. Med., 30(3), 561-568.
2. Kwee, T. C., et al. (2009). Magn. Reson. Mater. Phys. 22(5), 319-325.
3. Liau, J., et al. (2012). J. Magn. Reson. Med., 35(2), 318-327.
4. Nasu, K., et al. (2007). Magn. Reson. Mater. Phys., 20(4), 205.
5. Q. Nguyen, et al. (1998). Proc. ISMRM 0134.
6. Q. Nguyen, et al. (1999). Proc. ISMRM 0559.
7. Porter, D. A., et al. (2009). Magn. Reson. Med., 62(2), 468-475.
Figures