2711
Radiomics analysis for preoperative prediction of synchronous distant metastasis in patients with rectal cancer1Radiology, Xinhua hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China, 2Philips Healthcare, Shanghai, China
Synopsis
Rectal cancer is one of the most common malignant tumors in gastrointestinal tract. Tumor metastasis is still a major cause of death in patients with rectal cancer. The distant metastasis rate for rectal cancer remains constant at 20-50%1. Prediction of synchronous distant metastasis is important for the choice of personalized treatment strategies. Radiomics can extract quantitative features from digital images, which are related to the underlying pathophysiology2. We developed a radiomics model based on the MR radiomics features in combination with independent clinico-radiologic risk factors, which help to predict the synchronous distant metastasis in patients with rectal cancer.
Purpose: Rectal cancer is one of the most common malignant tumors in the world. Distant metastasis in patients with rectal cancer remains a problem influencing prognosis. Prediction of synchronous distant metastasis is important for the choice of personalized treatment strategies, such as metastasectomy or intensified systemic therapy3. Radiomics, which is based on advanced pattern recognition tools, involves the extraction of a large number of quantitative features from digital images to determine relationships between such features and the underlying pathophysiology. The aim of our study was to investigate the value of radiomics analysis based on MRI for preoperative prediction of synchronous distant metastasis in patients with rectal cancer.
Materials and methods: A total of 177 patients with histopathologically confirmed rectal cancer who underwent high-resolution MR imaging and contrast-enhanced chest-abdomen CT examinations before any treatment were enrolled in the study from October 2015 to August 2017. Axial, sagittal, and coronal T2-weighted MRI images were obtained using a fast spin-echo sequence (TR/TE, 3300-4000/104 msec; flip angle, 90°; echo train length, 16; field of view, 24×24 cm; matrix, 384×224; thickness, 3mm; intersection gap, 1 mm). Patients were divided into the primary cohort (n=123) and the validation cohort (n=54). The regions of interest (ROIs) were created manually via the itk-SNAP software (www.itksnap.org) based on the axial T2W image referring to the sagittal and coronal T2W images. Radiomics features were extracted using the AK software. Clinico-radiologic factors included gender, age, carcinoembryonic antigen level, CA199 level, MR-predicted T status (mrT), and MR-predicted lymph nodes (mrN) status. The independent two-sample t test, Kruskal-Wallis test, and Pearson correlation analysis were used for features selection. A radiomics signature was built and multivariable logistic regression analysis was used to develop the radiomics model including radiomics features and independent clinico-radiologic risk factors. The performance of the radiomics model was assessed by its calibration, discrimination, and clinical usefulness.
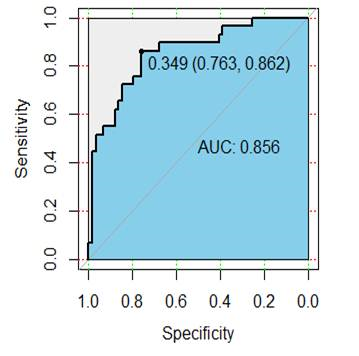
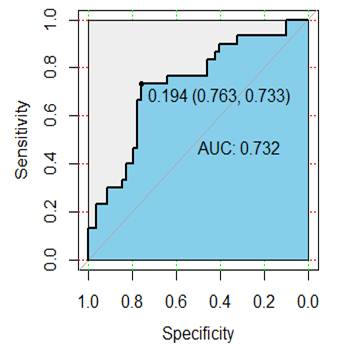
Results: Among the patients, fifty-nine patients were confirmed to have synchronous distant metastasis, including 37 with liver metastasis, 15 with lung metastasis, 5 with liver and lung metastasis, and 2 with bone metastasis. A total of 385 radiomic features were extracted from each patient, and 9 radiomics features (uniformity , GLCMEnergy, GLCMEntropy, Correlation, differenceEntropy , inverseDifference- Moment, HighGreyLevelRunEmphasis, LongRunLowGreyLevel- Emphasis, SurfaceVolumeRatio) were selected for the radiomics signature. Among the clinico-radiologic factors, mrN status and CA199 were independent risk factors for the synchronous distant metastasis. The radiomics model included radiomics signature, mrN status, and CA199. The model showed good performance in the primary cohort and validation cohort, the areas under the receiver characteristic curve were 0.856 (95% confidence interval (CI), 0.768-0.912) and 0.752 (95% CI, 0.648-0.916), respectively (Fig.1-2). Decision curve analysis confirmed the clinical utility of the radiomics model.
Discussion: Recently, machine learning-based approaches through analyzing large numbers of medical image features (called radiomic features) have been identified as a potential alternative to conventional methodologies for lesion detection and classification. Radiomics analysis integrates many high-dimensional imaging features that are difficult to detect visually. The computer-extracted texture features could help to quantitatively describe tissue microarchitecture. In the present study, 9 radiomics features were selected through dimension reduction. We established a radiomics model including radiomics signature and independent clinico-radiologic factors. The model showed good performance, indicating the feasibility of radiomic analysis in the prediction of synchronous distant metastasis.
Conclusion: We developed a radiomics model based on the MR radiomics features in combination with independent clinico-radiologic risk factors. The model could be used to predict the synchronous distant metastasis in patients with rectal cancer, which could be helpful for individual treatment strategy.
Acknowledgements
No acknowledgement found.References
1. Rasanen M, Carpelan-Holmstrom M, Mustonen H, et al. Pattern of rectal cancer recurrence after curative surgery. Int J Colorectal Dis. 2015; 30:775-785.
2.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012 Mar;48(4):441-6.
3.Sohn B, Lim JS, Kim H, et al. MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol. 2015; 25:1347-1355.