2704
Simultaneous Multi-Contrast Imaging in Combination with in-plane Parallel Imaging1MR Physics, Fraunhofer MEVIS, Bremen, Germany, 2University Bremen, Bremen, Germany, 3mediri GmbH, Heidelberg, Germany, 4MRI Physics, Imaging Centre of Excellence, College of Medical, Veterinary & Life Sciences, University of Glasgow, Glasgow, Scotland
Synopsis
Simultaneous Multi-Contrast (SMC) Imaging enables a synchronous acquisition of multiple image contrasts within one measurement. The technique reduces patient examination times and facilitates accurate image registration between contrasts. Previous work used readout-segmented EPI (rs-EPI) to perform high-resolution, navigator-corrected, diffusion-weighted imaging simultaneously with a T2*-weighted acquisition. This combination of contrasts has clinical significance in acute stroke. These previous studies did not use in-plane acceleration to reduce spatial distortion caused by the EPI readout. This study introduces an updated version of the SMC technique that incorporates in-plane acceleration with GRAPPA to allow an improved image quality for future clinical studies.
Purpose
Simultaneous Multi-Contrast (SMC) Imaging1,2 uses methodology from simultaneous-multi-slice (SMS) imaging to enable the acquisition of multiple image contrasts within a single measurement. Initial application has focused on the synchronous acquisition of diffusion-weighted (DW) T2*-weighted (T2*W) images using a readout-segmented (rs) echo planar (EPI) imaging sequence with navigator-based phase correction3. This has particular clinical relevance for acute stroke, where examination time is a critical factor4. A limitation to previous studies was that in-plane parallel imaging was not used, resulting in an EPI readout with a long effective echo spacing, leading to increased spatial distortion. The purpose of the current study was to combine the SMC technique with in-plane parallel imaging using GRAPPA5 to achieve an improved image quality that is suitable for clinical studies.Methods
Pulse Sequence:
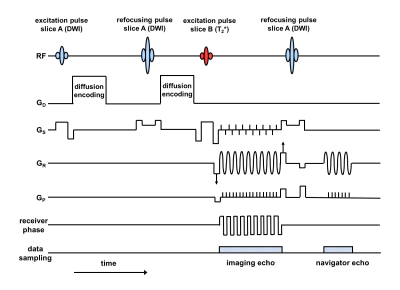
The modified rs-EPI sequence for SMC (Fig. 1), acquires DW and T2*W contrast from two slice positions at the same time. A blipped-CAIPIRINHA6,7 gradient scheme along the slice-select (GS) direction is used in conjunction with receiver phase modulation to shift the T2*-weighted image by half a field of view (FOV) in the phase-encoding direction. Finally, an RF refocusing pulse is applied to slice A only to generate a 2D navigator signal to phase correct the DW imaging data. The sequence was used firstly without in-plane acceleration and then with acceleration factors (AF) of 2 and 4 to sample every second or every fourth phase-encoding line respectively.
Data Acquisition:
Data were acquired from a healthy subject at 3T (MAGNETOM Skyra, Siemens Healthcare GmbH, 20 channel-head coil) with the following parameters: FOV 230mm, matrix 224x224, slice thickness 4mm, TR 4500ms; (DW contrast) one scan at b-value of zero and three scans at 1000s/mm2 along phase-encode, readout and slice-select directions; (T2*W contrast) flip angle 30°. Separate multi-slice reference data were acquired for DW contrast with b=0 and for T2*W contrast for the SMC reconstruction. In addition, low-resolution reference scans were acquired for the in-plane GRAPPA5 reconstruction.
Data Processing:
The slice-GRAPPA reconstruction7
with inter-slice leakage artefact reduction8 was used to separate
the aliased slices into single-slice data. Firstly, the SMC reference data were
used to fit separate 3 x 3 kernels. The same weight sets were used at all
b-values to separate DW and T2*W images. Secondly, the separated data were
passed to the standard in-plane GRAPPA reconstruction5. All data
processing was performed using the manufacturer’s proprietary image calculation
environment (ICE).
Results
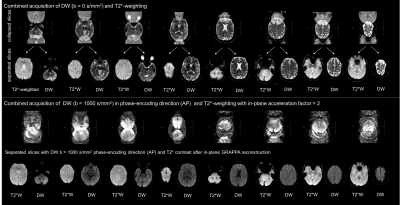
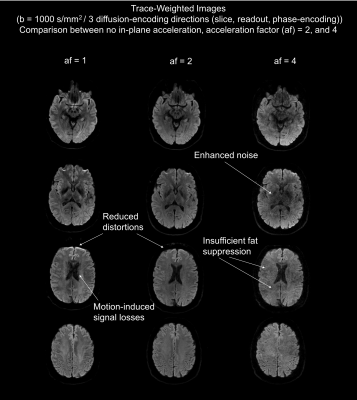
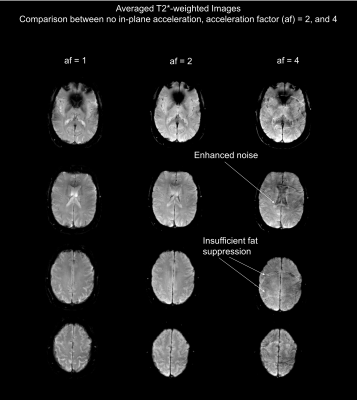
The top row of Fig. 2 shows the result of the simultaneous acquisition of DW data with a b=0 and T2*W data from a separate slice position without in-plane acceleration. The separated contrasts are displayed in the second row. The lower section shows the result of an SMC acquisition with an in-plane AF of two for a single diffusion-encoding direction and a b-value of 1000 s/mm2. Fig. 3 compares the result of calculated trace-weighted images without in-plane acceleration to those with an AF of two and four. Fig. 4 shows the corresponding T2*W data with three averages.Discussion
The separated images in Figs. 2, 3, and 4 demonstrate the feasibility of combining SMC with in-plane acceleration. Signals from the two contrast types are correctly assigned to the correct slice positions without evidence of cross talk. A high level of signal separation was achieved for both low and high b-value scans. The benefit of using GRAPPA to reduce the EPI echo-train length is clearly demonstrated by the reduced distortions in the images with in-plane acceleration shown in Fig. 3. However, the images with an AF of four also show an unacceptable loss in SNR. Fat suppression was performed by slice-select-gradient polarity reversal for DW signals and by water excitation for T2*W signals. In both cases these techniques proved to be inadequate at high in-plane acceleration factors, which requires attention in future work. Further work is also required to address the motion-induced signal losses seen in some images because the current implementation of the sequence does not use a reacquisition scheme to avoid uncorrectable, severe phase errors, as described in previous studies using DW rs-EPI3.Conclusion
This study has demonstrated that SMC can be combined with in-plane parallel imaging to allow the simultaneous acquisition of DW and T2*W images with reduced spatial distortion. The resulting technique promises to be of particular interest for reducing overall measurement times in acute stroke when TR values are too short for SMS techniques to be used. In addition, the method provides DW and T2*W images that are inherently co-registered with respect to both subject motion and residual distortions caused by the EPI readout.Acknowledgements
The Authors are grateful to Dr Robert Frost and the Welcome Centre For Integrative Neuroimaging at Oxford University for providing source code for their Slice-Grappa implementation. Thanks are also due to Klaus Eickel for helpful discussions during the course of this work.References
1. Breutigam NJ, Frost R, Eickel K, Porter DA. Simultaneous Multi-Contrast Imaging with Readout-Segmented EPI. In Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, USA, 2017. Abstract 0520.
2. Breutigam NJ, Frost R, Eickel K, Porter DA. Simultaneous Multi-Contrast (SMC) Imaging for Synchronous DWI and T2*-Weighting. In Proceedings of the 34th Annual Meeting of ESMRMB, Barcelona, Spain, 2017. Abstract 859.
3. Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med. 2009;62(2):468–75.
4. Schellinger PD, Jansen O, Fiebach JB, Hacke W0, Sartor K. A Standardized MRI Stroke Protocol Comparison with CT in Hyperacute Intracerebral Hemorrhage. Stroke 1999;30:765-768.
5. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B and Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 2002;47:1202-10.
6. Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled Aliasing in Parallel Imaging Results in Higher Acceleration (CAIPIRINHA) for Multi-Slice Imaging. Magn Reson Med 2005;53:684-691.
7. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med 2012;67:1210-1224.
8. Cauley SF, Polimeni JR, Bhat H, Wald LL, Setsompop K. Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. Magn. Reson. Med. 2014;72(1):93-102.
Figures