2689
KT-Points Pulses Reduce B1 Shading at 3T: Demonstration in Routine Abdominal DCE-MRI and Evaluation of Reliability1CEA/DRF/Joliot/NeuroSpin/UNIRS, Gif-sur-Yvette, France, 2Siemens Healthcare SAS, Saint-Denis, France, 3Department of Radiology, AP-HP, CHU Henri Mondor, Créteil, France, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Université Paris-Est Créteil Val-de-Marne, Créteil, France, 6INSERM Unité U955, Equipe 18, Créteil, France
Synopsis
At high field, MRI systems offer a higher signal-to-noise ratio, but B1+-inhomogeneity-induced artefacts in large organs can lead to shading and erroneous contrast. In this work, subject-tailored kT-points pulse design performance was evaluated in clinical routine on liver DCE-MRI at 3T, against that of patient-specific RF shimming. Both excitation homogeneity simulation and image quality assessment were performed on a variety of patients. The interest of kT-points is clearly demonstrated, as well as the reliability of the approach.
Introduction
The gain in signal-to-noise ratio brought by high-field MRI can be hampered by zones of shading and loss of contrast, caused by dielectric resonance in large organs.1 With the advent of two-transmit-channel commercial scanners and automated B1+-mapping calibration, patient-tailored static RF shimming has stood out, in clinical routine, as a standard method to reduce this artefact.2–4 The present work follows a pilot study5 on B1+ inhomogeneity mitigation in the abdomen using kT-points.6,7 Here, the context is Dynamic Contrast-Enhanced (DCE) MRI, an important part of standard liver examination protocols8: after contrast agent injection, a succession of 3D fat-saturated T1-weighted GRE acquisitions allows to follow the perfusion of tissues. An inhomogeneous B1+ field can cause inaccurate enhancement, leading to fallacious tissue and lesion characterisation. This study aims at evaluating excitation homogeneity and image quality achieved by prototypical kT-points pulses compared to tailored static RF shimming, in clinical routine DCE-MRI on a dual-transmit 3T scanner, and to assess the reliability of the technique.Methods
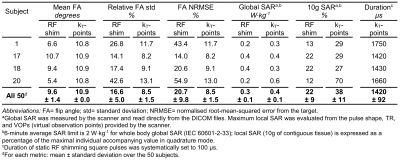
Acquisitions were carried out on a MAGNETOM Skyra 3T scanner (Siemens Healthcare, Erlangen, Germany), equipped with a product two-transmit-channel system, on 50 consecutive patients referred for liver MRI. For each subject, B1+ field maps for each transmission channel and a Δf0 (Larmor frequency offset) map were acquired. Flip angle (FA) map simulations based on these maps were performed via numerical integration of Bloch's equation: the FA average, relative standard deviation and normalised root-mean-square error (NRMSE) in the imaged volume were estimated for both static RF shimming and kT-points pulses. B1+ field maps were obtained from the manufacturer standard adjustment procedure used for patient-specific static RF shimming, along with the virtual observation points9 (VOP) needed for SAR calculation. Δf0 maps were measured using a two-echo GRE breath-hold acquisition, with ΔTE= 0.95ms, short enough to avoid temporal phase wrapping.
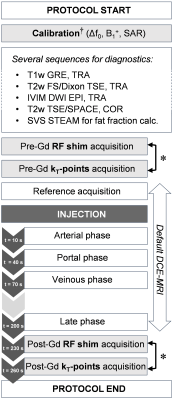
20 of the 50 patients underwent 3D breath-hold DCE-MRI while a pulse designer was present. RF shimming and kT-points pulses could be compared by repeating the acquisition with each transmit scheme before injection and in the late phase – in light grey on Figure 1 – with the same sequence parameters: FA= 11°, TE/TR= 3/6ms, 320×220×72 matrix, 1.2×1.2×3.5mm3 resolution, GRAPPA factor 2, 80%/50% phase/slice resolution, partial-Fourier factor of 6/8, 505Hz/pixel bandwidth, "quick" fat saturation10. Relative contrast enhancement (rCE) was evaluated for both techniques:
$$\mathrm{rCE}=\frac{S_{after}-S_{before}}{S_{before}} \times 100\%$$
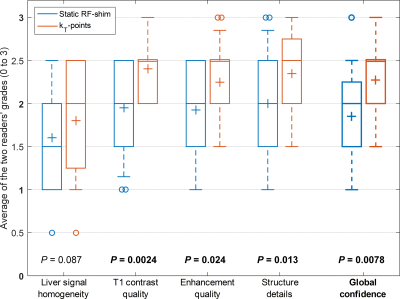
where $$$S_{before}$$$ and $$$S_{after}$$$ represent signal before and after injection. In addition, signal homogeneity, T1 contrast, enhancement quality, structure details and global image quality were qualitatively assessed on a four-level scale (0 to 3) by two independent radiologists.
RF shimming was performed with 100-µs square pulses, complex coefficients being automatically calculated by the scanner for every subject. On the other hand, a 9-kT-point pulse was computed in less than one minute using MATLAB’s (The Mathworks, Natick, MA, USA) built-in active-set algorithm on a laptop computer. FA NRMSE was minimised by optimising simultaneously RF coefficients, kT‑point locations, and durations under SAR and hardware constraints.11–13
As fat-selective saturation was performed10,14, it was not necessary to target a specific FA in fat voxels. Water was discriminated from fat using Δf0 maps, and only water voxels were considered during pulse design and for FA homogeneity assessment. For quantitative results and for each aspect of image quality, a matched-pairs one-tailed Wilcoxon signed-rank test was computed using the R (www.r-project.org) exactRankTests package15 to compare both techniques. All P-values were considered statistically significant when less than 0.05.
Results
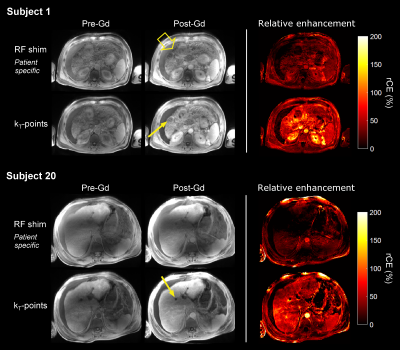
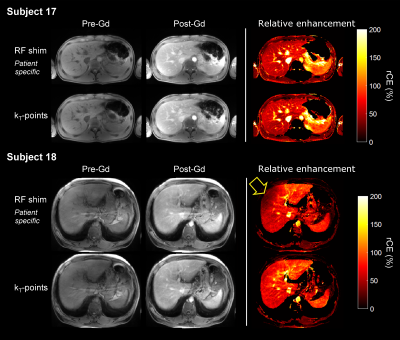
As shown in Table 1, FA NRMSE was significantly reduced with kT-points compared to static RF shimming (8.5% ± 1.5% vs 20.7% ± 9.8%; P < 0.0001). The worst case was 13.0% (kT-points) vs 54.9% (RF shimming). Figures 2 and 3 show examples of acquired anatomical images (pre- and post-injection), with the corresponding rCE maps. Qualitative grades obtained with kT-points were higher than with static RF shimming for all criteria (Figure 4). Global image quality was significantly higher for kT-points (2.3 ± 0.5 vs 1.9 ± 0.6; P = 0.008). One patient's examination (subject 20) was judged unusable with RF shimming by one reader, none with kT-points. 85% of kT-points acquisitions were graded at least 2/3, and only 55% for static RF shimming.Conclusion
The reliability of the kT-points pulse design in a clinical context was demonstrated on a large group of various patients. KT-points reduce excitation inhomogeneity quantitatively and qualitatively, especially in patients with ascites and prone to B1 shading. These first results in DCE-MRI promise great applications to other anatomical regions at 3T, be it for non-selective preparation or for 3D imaging.Acknowledgements
The authors wish to thank all the MRI technicians of Henri Mondor Hospital for their patience, understanding and helpful clinical advices. This project was funded by CEA’s Programme Transversal, Technologies pour la Santé (Transversal Programme for Health Technologies).References
1. Bernstein MA, Huston J, Ward HA. Imaging artifacts at 3.0T. J Magn Reson Imaging. 2006 Oct 1;24(4):735–46.
2. Kukuk GM, Gieseke J, Nelles M, König R, Andersson M, Muschler E, et al. Clinical liver MRI at 3.0 Tesla using parallel RF transmission with patient-adaptive B1 shimming. In: Proc Intl Soc Mag Reson Med [Internet]. 2009 [cited 2016 Oct 4]. p. 119. Available from: http://cds.ismrm.org/protected/09MProceedings/files/00119.pdf
3. Brink WM, Gulani V, Webb AG. Clinical applications of dual-channel transmit MRI: A review: Clinical Applications of Dual-Channel Transmit MRI. J Magn Reson Imaging. 2015 Oct;42(4):855–69.
4. Padormo F, Beqiri A, Hajnal JV, Malik SJ. Parallel transmission for ultrahigh-field imaging. NMR Biomed. 2016 Sep 1;29(9):1145–61.
5. Tomi-Tricot R, Gras V, Mauconduit F, Legou F, Boulant N, Gebhardt M, et al. SAR-Constrained kT-Points Pulse Design Applied to B1 Inhomogeneity Mitigation in the Human Abdomen at 3T. In: Proc Intl Soc Mag Reson Med [Internet]. Honolulu, HI, USA; [cited 2017 Oct 25]. p. 4830. Available from: http://indexsmart.mirasmart.com/ISMRM2017/PDFfiles/4830.html
6. Amadon A, Cloos MA. Method and apparatus for compensating for B1 inhomogeneity in magnetic resonance imaging by nonselective tailored RF pulses. WO2011/128847A1, 2011.
7. Cloos MA, Boulant N, Luong M, Ferrand G, Giacomini E, Le Bihan D, et al. kT-points: Short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magn Reson Med. 2012 Jan;67(1):72–80.
8. Kierans AS, Leonardou P, Shaikh F, Semelka RC. Body MR imaging: Sequences we use and why. Appl Radiol. 2009;38(5):7–12.
9. Eichfelder G, Gebhardt M. Local specific absorption rate control for parallel transmission by virtual observation points. Magn Reson Med. 2011 Nov 1;66(5):1468–76.
10. Le Y, Kroeker R, Kipfer HD, Lin C. Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J Magn Reson Imaging. 2012 Aug 1;36(2):483–91.
11. Gras V, Luong M, Amadon A, Boulant N. Joint design of kT-points trajectories and RF pulses under explicit SAR and power constraints in the large flip angle regime. J Magn Reson. 2015 Dec;261:181–9.
12. Hoyos-Idrobo A, Weiss P, Massire A, Amadon A, Boulant N. On variant strategies to solve the magnitude least squares optimization problem in parallel transmission pulse design and under strict SAR and power constraints. IEEE Trans Med Imaging. 2014;33(3):739–748.
13. Tomi-Tricot R, Gras V, Boulant N, Vignaud A, Amadon A. kT-Points Pulse Design at 7T: Optimization of Pulse and Sub-Pulse Durations. In: Magnetic Resonance Materials in Physics, Biology and Medicine [Internet]. Vienna, AT; 2016 [cited 2016 Oct 27]. p. 247–400. Available from: http://link.springer.com/10.1007/s10334-016-0570-3
14. Leyendecker JR, Gakhal M, Elsayes KM, McDermott R, Narra VR, Brown JJ. Fat-suppressed dynamic and delayed gadolinium-enhanced volumetric interpolated breath-hold magnetic resonance imaging of cholangiocarcinoma. J Comput Assist Tomogr. 2008 Apr;32(2):178–84.
15. Hothorn T, Hornik K. exactRankTests: Exact Distributions for Rank and Permutation Tests [Internet]. 2017. Available from: https://CRAN.R-project.org/package=exactRankTests
Figures