2683
PET/MR dynamic imaging of an inflatable phantom with self-gated UTE-MRI1Imagerie par Résonance Magnétique Médicale et Multi-Modalités, IR4M, CNRS, Univ. Paris Sud, Orsay, France, 2Imagerie Moleculaire In Vivo, IMIV Laboratory, CEA, Orsay, France, 3Applications & Workflow, GE Healthcare, Orsay, France, 4Department of Radiology and Biomedical Imaging, University of California - San Francisco, San Francisco, CA, United States
Synopsis
MRI offers many advantages for chest imaging such as the absence of irradiation and the opportunity to obtain images with various contrasts in soft tissues. Developing MRI lung imaging would provide solutions to a real public health problem related to lung disease. Besides, PET is relevant for the study of metabolic changes caused by parenchymatous affections. Hence PET-MRI is a promising route for the characterization of lung diseases. One of the immediate issues lung imaging raises is motion. Physiological motion needs to be taken into account during the imaging process to avoid blurring or ghosting artifacts in both imaging modalities.
Purpose
The first purpose of this work was to design and conceive a hydraulically controlled dynamic phantom for PET/MR imaging simulating the lung function. Three radioactive Germanium spheres detectable in PET were fixed on the phantom to realize the first dynamic PET/MR acquisitions.
The second purpose of this work was to acquire and reconstruct the PET and MRI images of this phantom with the correction of artifacts caused by the periodic motion of inflation and deflation of the latex balloon, simulating here the lung, in order to obtain a bi-modal image at different points of the inflation-deflation cycle.
Materials & Methods
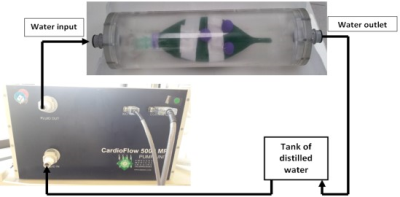
To mimic the lung respiratory motion, a phantom was designed with a latex balloon enclosed in a cylinder filled with water – to be detected with MRI – and marked with three radioactive Germanium spheres (0.1 MBq) – to be detected with PET. The latex balloon inflation was hydraulically controlled by an external pump (CardioFlow 5000 MR, Programmable Physiological Flow Pump by SHELLEY Medical Imaging Technologies, London, Ontario CANADA) which imposed oscillating water flows in the cylindrical enclosure, hence forcing the balloon to breath in and out air (Fig. 1). The enclosed water surrounding the balloon played a similar role as the diaphragm during breathing for the lungs.
To account for very short relaxation times (T2 and T2*) expected in the lungs, a UTE pulse sequence with radial sampling (1) (2) was implemented on a hybrid PET/MR 3.0 T system (GE Healthcare, Milwaukee, WI, USA). The acquisition parameters were TE = 100 μs, TR =5.33 ms, BW = 125 kHz, FOV = (120×120×324) mm3, pixel size = (1×1×1) mm3, number of excitations = 3, total acquisition time = 5 min 21 s. The MRI acquisition was performed simultaneously over a 6 min PET acquisition.
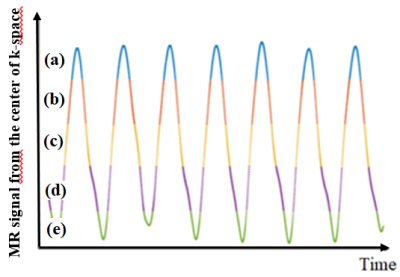
To account for motion in the MR images, a self-gating method was applied(3) . This involved (1) the extraction of the signal at the k-space center, (2) the selection of 5 respiratory phases, each one containing approximatively 20% of the signal, and (3) the application of 3D inverse NUFFT for each gate (Fig. 2). To account for motion in the PET images, the five respiratory phases selected for MRI were used to generate five corresponding list-mode data sets out of the original list-mode data. Motion-phased PET images were then separately reconstructed with CASToR(4) from the 5 list-mode data sets. Finally, the five MR and PET images were overlaid.
Results & Discussion
The respiratory trace of the latex balloon extracted from the k-space
center signal is represented on Fig. 2 with outlined selected motion
phases. The signal decreases when the balloon is inflating, namely being
filled with air. It increases when the balloon is deflating, namely
when the water volume increases in the enclosure. An axial slice of the
overall MR and PET images before motion-phasing is presented on Fig. 3.
As expected for a 6 min acquisition, motion blurring is obvious and
germanium spheres do not appear spherical anymore in both modalities (in
positive contrast for PET and in negative contrast for MRI). Axial and
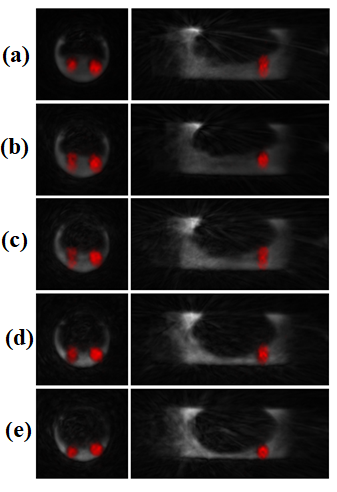
sagittal slices of fused MR and PET images after motion-phasing are
presented on Fig. 4. Even though some distortion remains in the
rapidly-changing phases (between total inflation and deflation (b) and
(c) on Fig. 4), the five motion-phased bi-modal images corresponding to
the five selected gates show much better defined anatomy and the
germanium spheres generally appear more spherical.Conclusion
The reduction of respiratory motion artifacts in phased MR
and PET images show the robustness of the MR self-gating method for PET-MR
imaging. Increasing the number of phases would lead to better
spatially-resolved images but a compromise should be found with the
corresponding SNR reduction.Acknowledgements
IR4M Laboratory, IMIV Laboratory. Imaging was performed on the GE PET-MRI platform at SHFJ (Orsay, France).
References
1. MR imaging with ultrashort TE (UTE) pulse sequences: Basic principles. Joanne E.Holmes, Graeme M.Bydder. August 2005, Radiology, pp. Volume 11, Issue 3.
2. Detection of Small Pulmonary Nodules with Ultrashort Echo Time Sequences in Oncology Patients by Using a PET/MR System. Nicholas S. Burris MD, Kevin M. Johnson PhD, Peder E. Z. Larson PhD, Michael D. Hope MD, Scott K. Nagle MD, PhD, Spencer C. Behr MD, Thomas A. Hope, MD. January 2016, Radiology, p. Volume 278: Number 1.
3. Self-gated Radial MRI for Respiratory Motion Compensation on Hybrid PET/MR Systems. Robert Grimm, Sebastian Furst, Isabel Dregely, Christoph Forman, Jana Maria Hutter, Sibylle I. Ziegler, Stephan Nekolla, Berthold Kiefer, Markus Schwaiger, Joachim Hornegger, Tobias Block. 2013.
4. Thibaut Merlin, Simon Stute, Didier Benoit, Julien Bert, Thomas Carlier, Claude Comtat, Frédéric Lamare and Dimitris Visvikis. CASToR : A Generic Data Organization and Processing Code Framework for Multi-Modal and Multi-Dimensional Tomographic Reconstruction. [Poster] 2016.
5. Motion Correction Options in PET/MRI. Ciprian Catana, MD, PhD. 2015, Nuclear Medecine, pp. Semin Nucl Med 45:212-223.
Figures