2682
Radius Segmented Multi-shot Spiral for Diffusion Imaging1Research & Development Group, Hitachi, Ltd., Tokyo, Japan, 2Healthcare Business Unit, Hitachi, Ltd., Chiba, Japan
Synopsis
Single-shot echo-planar imaging (EPI) is usually used in diffusion-weighted imaging (DWI); however, it is difficult to apply to examining the entire brain because of image distortion due to susceptibility inhomogeneity. In addition, multi-shot imaging, in which image distortion is relatively small, is affected by pulsation artifacts and aliasing. We propose a multi-shot spiral method in which a spiral trajectory is divided in the radial direction. DWI studies were performed on the brain of a healthy volunteer. The proposed method could sample k-space data for each shot without aliasing, and sufficient correction for pulsation artifacts could be obtained.
Purpose
Imaging methods that do not produce distortion are important for DWI targeting the whole brain. Although multi-shot DWI is an advantageous method for obtaining images without distortion, these images contain artifacts induced by pulsation. A method for correcting the phase error in the image domain to reduce the number of pulsation artifacts was reported [1]. However, aliasing artifacts due to under-sampling prevent phase correction because the aliased components require the same phase as the unaliased components at any location to correct for the phase error. The aim of this work is to provide a multi-shot spiral imaging method that can reduce the number of aliasing artifacts and consequently reduce the number of pulsation artifacts.Methods
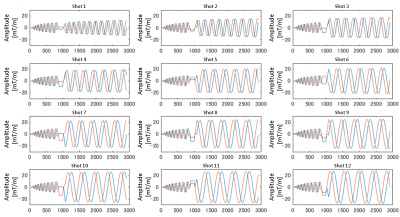
In this study, we propose a multi-shot spiral method in which a single-shot spiral trajectory is divided in the radial direction and sampled for each shot along the divided trajectories. Figure 1 shows the k-space trajectories for 12 shots, and Fig. 2 shows the corresponding spiral waveforms. Since the sampling density of each shot is the same as that of single-shot, the Nyquist criterion is always satisfied, and consequently, the number of aliasing artifacts can be reduced. Moreover, pulsation artifacts can be removed by sampling the center area of k-space in every shot and by correcting the phase error in the center area. All images were obtained with a 3.0-T scanner that uses a 15-channel receiver coil, and the main parameters were TR/TE = 1000 ms /87 ms, 12 interleaves, FOV = 250 mm, and pixel size = 160 × 160 (output image size = 256 × 256). The image processing in this study is described below. First, spiral data were resampled on Cartesian k-space by using a gridding method shot-by-shot. Second, phase conjugate maps in image space from the center part of the k-space data were calculated. Third, the image obtained for each shot was multiplied by the phase conjugate. Last, corrected images were collected for every shot.Results
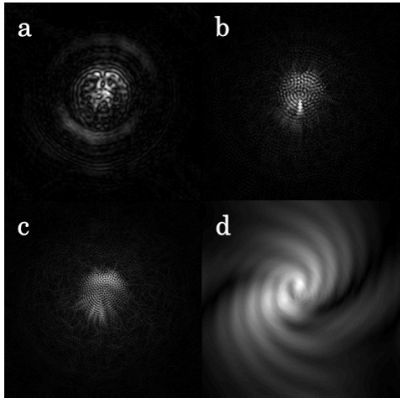
Figure 3 shows diffusion-weighted images acquired with the proposed spiral sequence with b = 1000 sec/mm2. The diffusion gradient directions were [1 0 0], [0 1 0], and [0 0 1]. Due to the effect of the pulsation, the image quality was severely deteriorated in the images without motion correction, as shown in Figs. 3(a)-(b). In comparison, in the images shown in Figs. 3 (c)-(d), the pulsation artifacts were removed by the phase correction, and the image quality improved. Moreover, there were few aliasing artifacts in these images. Since the influence of pulsation artifacts is negligible in the case of imaging without diffusion-sensitizing gradients, images can be reconstructed without phase correction in the image domain. However, in diffusion-weighted multi-shot imaging, diffusion-sensitizing gradients enhance the influence of motion, so the phase variation among shots is not negligible. In this case, motion-induced phase errors must be removed before combining the k-space data of all shots. In the conventional multi-shot (interleaved) spiral imaging, the outer part of k-space for each shot is sampled below the Nyquist rate. This leads to severe aliasing artifacts, as shown in Fig. 4(d). To remove the artifacts, synthesizing the missing data by using multi-coil data is possible [2]; however, this leads to an increase in computational cost. In comparison, the proposed multi-shot spiral method, in which the trajectory is divided in the radial direction, can sample k-space data for each shot without under-sampling and consequently suppress aliasing artifacts in image data, as shown in Figs. 4 (a)-(c). Therefore, sufficient phase correction can be obtained in the image domain without increasing computational costs.Discussion & Conclusion
The feature of the proposed method is that the sampling interval does not change even if the shot number is increased. This is advantageous in that aliasing artifacts can be suppressed. However, one disadvantage is that the effect of reducing image distortion with susceptibility inhomogeneity is limited in comparison with an interleaved-type method that can reduce distortion by widening the sampling interval in the phase direction. Therefore, to reduce image distortion, it is necessary to shorten the sampling time as much as possible in the proposed method. Since the center of k-space is oversampled in the spiral trajectory, our method is basically insensitive to motion compared with EPI-based methods such as RESOLVE or PROPELLER [3-4]. Moreover, the center data can be used for the phase correction as self-navigation data. In summary, our method can be a powerful alternative to the conventional multi-shot DWI methods.Acknowledgements
No acknowledgement found.References
1. Liu C, Bammer R, Kim D, Moseley ME. Self-Navigated Interleaved Spiral (SNAILES): Application to High-Resolution Diffusion Tensor Imaging. Magn Reson Med 2004; 52: 1388-1396.
2. Liu C, Moseley Me, Bammer R. Simultaneous Phase Correction and SENSE Reconstruction for Navigated Multi-Shot DWI with Non-Cartesian k-Space Sampling. Magn Reson Med 2005; 54: 1412-1422.
3. Porter DA, Heidemann RM. High Resolution Diffusion-Weighted Imaging Using Readout-Segmented Echo-Planar Imaging, Parallel Imaging and a Two-Dimensional Navigator-Based Reacquisition. Magn Reson Med 2009; 62: 468-475.
4. Pipe JG. Motion Correction with PROPELLER MRI: Application to Head Motion and Free-breathing Cardiac Imaging. Magn Reson Med 1999; 42: 963-969.
Figures