2674
Development of a spiral spin- and gradient-echo (spiral-SAGE) approach for improved dynamic contrast neuroimaging1Translational Bioimaging Group, Barrow Neurological Institute, Phoenix, AZ, United States, 2Magnetic Resonance Technology Design Group, Barrow Neurological Institute, Phoenix, AZ, United States, 3Phoenix Children's Hospital, Phoenix, AZ, United States
Synopsis
The purpose of this study is to develop a spiral-based combined spin- and gradient-echo (spiral-SAGE) pulse sequence for simultaneous dynamic contrast-enhanced (DCE-MRI) and dynamic susceptibility contrast MRI (DSC-MRI). Using this sequence, we obtained gradient-echo TEs of 1.69 and 26 ms, a SE TE of 87.72 ms, with a TR of 1663 ms. Using an iterative SENSE reconstruction followed by deblurring, spiral-induced image artifacts were minimized. Comparison of spiral-SAGE images with conventional EPI-SAGE images illustrates substantial improvements in image distortion and image intensity variations. Spiral-SAGE provides a significant improvement for the assessment of perfusion and permeability in various neuropathologies.
Introduction
DSC-MRI provides valuable information related to brain perfusion, including cerebral blood volume, while DCE-MRI is the standard method for measuring vascular permeability. The SAGE sequence leverages multiple EPI readout acquisitions to provide both DSC and DCE information within a single acquisition [1–5]. While EPI readouts provide high temporal resolution for DSC-MRI at reasonable spatial resolution, the drawbacks to EPI include image distortion and intensity variations (including signal voids and signal pile-ups). In addition, the first SAGE TE is typically longer (>5 ms) than recommended for DCE-MRI [6], as the readout trajectory starts at the edge of k-space. Spiral-based readouts circumvent both of these issues, eliminating image distortion and providing shorter first TEs with a spiral-out trajectory [7]. Other advantages with spiral readouts include increased time efficiency, decreased motion sensitivity, and possibly more aggressive undersampling to improve temporal and/or spatial resolution. The purpose of this study is to develop a spiral-SAGE pulse sequence with an advanced reconstruction scheme and compare those images to standard EPI-SAGE images in a healthy volunteer.Methods
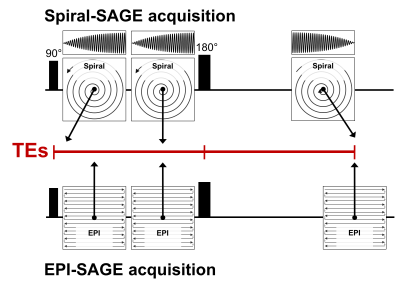
The spiral-SAGE sequence shown in Figure 1 was developed on the Philips platform. The trajectory for both gradient echoes was spiral-out, while the SE trajectory was spiral-in. As such, the center of k-space occurs at the beginning of the acquisition window for both gradient echoes and at the end of the acquisition window for the SE. Data were acquired at 3T (Ingenia, Philips Healthcare) in a healthy volunteer. The spiral-SAGE sequence provided the following imaging parameters: TEs = 1.69, 26.00, and 87.72 ms, TR = 1663 s, FOV = 240x240 mm2, 15 slices, and voxel size = 3.158x3.158x5 mm3. The spiral readout length (t) was 15.1 ms, and variable density undersampling was employed with the center 10% of k-space fully sampled and a maximum undersampling factor of 2.5. For comparison, the EPI-SAGE sequence provided TEs = 7.96, 26.02, and 79.61 ms and TR = 1.8 s, with matching spatial parameters. Spiral data were reconstructed off-line in GPI [8] using an iterative SENSE algorithm [9] based on a gridding reconstruction with sampling density weights calculated a priori from the spiral k-space coordinates [10]. Receive coil sensitivity maps were generated on the scanner by the vendor’s software. Following SENSE reconstruction, the images were deblurred using a convolution based deblurring algorithm [11] and B0 map generated from a separate Dixon acquisition. Using echoes 1 and 2, the signals were combined to isolate the T1-weighted signal (i.e., signal extrapolated to TE = 0, no T2* contribution) and R2* (no T1 contribution); using the signal extrapolated to TE = 0 and the SE, the R2 contribution was also isolated [12].Results/Discussion
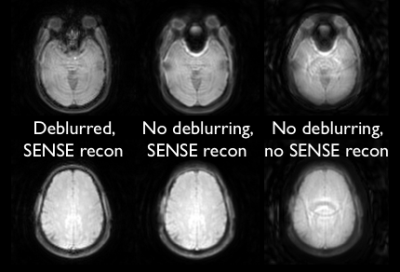
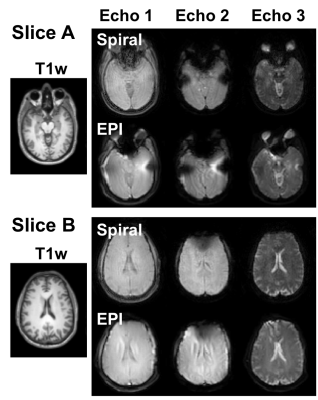
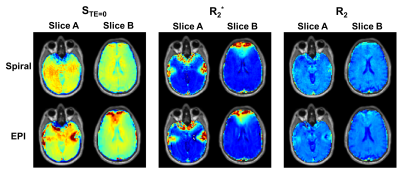
The effect of parallel imaging reconstruction and spiral deblurring are illustrated in Figure 2. Residual artifacts in the spiral data may be the result of the confounding effects of aliasing and spiral blurring on the distribution of artifacts. The SAGE images for all three echoes using spiral and EPI readouts are shown in Figure 3 for two slices in a healthy volunteer. The inferior slice (A) shows signal voids and pile-ups for EPI, particularly near air-tissue interfaces, which are mitigated with spiral trajectories. The superior slice (B) is also impacted by image distortion and intensity variations with EPI, which are improved by the use of spiral readouts. For DCE-MRI using EPI-SAGE, the T1-weighted signal is obtained from extrapolation to TE=0; due to the shorter first TE (~2 ms), spiral-SAGE would permit use of either the TE=0 signal or the first echo signal. There is a clear advantage for the use of spiral-SAGE in this case (Figure 4). As the second and SE TEs are closely matched for spiral-SAGE and EPI-SAGE, the advantage for DSC-MRI is less obvious, but the reduced distortion and intensity variations result in more consistent R2* and R2 images for spiral-SAGE. While the spiral-SAGE parameters were chosen to match EPI-SAGE for image quality comparison, spiral readouts provide a significant opportunity for optimization of image parameters for DSC and DCE-MRI. Work is ongoing to further optimize the spiral parameters for dynamic spiral-SAGE in patients with brain tumors.Conclusions
Multi-echo acquisitions permit the measurement of multiple distinct hemodynamic parameters in a single acquisition, which is highly advantageous for reducing scan times and contrast agent dose in neuroimaging. The use of spiral readouts for dynamic contrast imaging has several advantages over EPI readouts, including increased time efficiency, shorter first echo times, reduced motion sensitivity, and improved image quality. This sequence provides substantial flexibility to optimize contrast for both DSC- and DCE-MRI.Acknowledgements
This work was supported by Arizona Biomedical Research Commission (ADHS16-162414), NIH/NCI 2R01CA158079, and Philips Healthcare.References
[1] Schmiedeskamp H, Andre JB, Straka M,
Christen T, Nagpal S, Recht L, et al. Simultaneous perfusion and permeability
measurements using combined spin- and gradient-echo MRI. J Cereb Blood Flow
Metab 2013;33:732–43. doi:10.1038/jcbfm.2013.10.
[2] Skinner JT, Robison RK, Elder CP, Newton AT, Damon BM, Quarles
CC. Evaluation of a multiple spin- and gradient-echo (SAGE) EPI acquisition
with SENSE acceleration: applications for perfusion imaging in and outside the
brain. Magn Reson Imaging 2014;32:1171–80. doi:10.1016/j.mri.2014.08.032.
[3] Stokes AM, Skinner JT, Yankeelov TE, Quarles CC. Assessment of
a simplified spin and gradient echo (sSAGE) approach for human brain tumor
perfusion imaging. Magn Reson Imaging 2016;34:1248–55.
doi:http://dx.doi.org/10.1016/j.mri.2016.07.004.
[4] Mouridsen K, Bjornerud A, Farrar CT, Jennings D, Borra RJH,
Wen PY, et al. Vessel architectural imaging identifies cancer patient
responders to anti-angiogenic therapy. Nat Med 2013:1–8.
[5] Semmineh NB, Xu J, Skinner JT, Xie J, Li H, Ayers G, et al.
Assessing tumor cytoarchitecture using multiecho DSC-MRI derived measures of
the transverse relaxivity at tracer equilibrium (TRATE). Magn Reson Med
2015;74. doi:10.1002/mrm.25435.
[6] DCE MRI Quantification Profile. Quant Imaging Biomarkers
Alliance 2012.
[7] Paulson ES, Prah DE, Schmainda KM. Spiral Perfusion Imaging
With Consecutive Echoes (SPICE) for the Simultaneous Mapping of DSC- and
DCE-MRI Parameters in Brain Tumor Patients: Theory and Initial Feasibility.
Tomography 2016;2:295–307. doi:10.18383/j.tom.2016.00217.
[8] Zwart NR, Pipe JG. Graphical programming interface: A
development environment for MRI methods. Magn Reson Med 2015;74:1449–60.
doi:10.1002/mrm.25528.
[9] Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in
sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med 2001;46:638–51.
doi:10.1002/mrm.1241.
[10] Zwart NR, Johnson KO, Pipe JG. Efficient sample density
estimation by combining gridding and an optimized kernel. Magn Reson Med
2012;67:701–10. doi:10.1002/mrm.23041.
[11] Ahunbay E, Pipe JG. Rapid method for deblurring spiral MR
images. Magn Reson Med 2000;44:491–4.
doi:10.1002/1522-2594(200009)44:3<491::AID-MRM22>3.0.CO;2-Z.
[12] Stokes AM, Quarles CC. A simplified spin and gradient echo
approach for brain tumor perfusion imaging. Magn Reson Med 2016;75:356–62. doi:10.1002/mrm.25591.
Figures