2657
Retrospective motion correction of head motion using electromagnetic sensors1Radiology, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
Motion artifacts pose significant problems for the acquisition of MR images, especially in pediatric populations. In this work we developed a retrospective motion correction framework that uses motion information from two electromagnetic sensors attached to the forehead of subjects. We evaluated our retrospective motion correction strategy on 12 different cases and show that that motion traces from the EM tracker can be used to retrospectively improve image quality.
Introduction
MRI is inherently sensitive to motion, and motion induced artifacts can significantly degrade image quality. While the effects of motion can be reduced to an insignificant level when cooperative subjects are scanned, they remain a major problem during scans of non-cooperative subjects such as children1. It is common practice for a high percentage of children (age 4-7 years) to only be scanned when under anesthesia. Unfortunately, sedation and anesthesia in children can have substantial risks and also significantly increase the cost. A method that successfully reduces the effects of motion could potentially eliminate the need for sedation, resulting in both reduced costs and reduced risks to patient health. Some promising prospective and retrospective correction techniques have been suggested2-5 but the clinical adaptation has been limited. In this work we investigate the feasibility of using an electromagnetic tracker (Robin Medical Inc.) to estimate rigid body motion parameters, and use them to retrospectively correct for motion in T1-weighted structural scans.Methods
We modified a clinically used T1-weighted MPRAGE sequence to test our retrospective correction with real-time motion tracking using electromagnetic (EM) sensors. The MPRAGE sequence was modified with additional gradient activations to enable real-time tracking. Two EM sensors were attached to the forehead of each subject. The location and orientation of both sensors were updated every TR, from which 6 rigid-body motion parameters were estimated. The euclidean distance between the two sensors was used as a metric for skin motion and non-rigid motion, and only one of the sensors was used if this number varied more than 0.1mm. After obtaining written informed consent, four adult volunteers were scanned on a Siemens 3T TRIO scanner using a 32-channel head coil. The echo time of the scan was 2.17ms with a repetition time of 1.56s. The field of view was 256x256x176mm^3 with a matrix size of 256x256x176, resulting in an isotropic 1mm resolution in a 4 minute scan. Each volunteer was scanned multiple times, where in the first scan they were instructed to stay still (“no motion”) and in the rest of the scans they were instructed to change their head position with verbal cues either two, four or eight different times (“motion”). In total we acquired 12 MPRAGE volumes with different amounts of motion and 4 scans without motion to be used as a reference scan. Raw k-space data was extracted from the scanner and each k-space line (from each coil) was corrected for phase ramps (translation in image domain) and regridded in 3D k-space using NUFFT toolbox6 (rotation in image domain) using the estimated rigid-body motion parameters as suggested by Gallichan et al7. The Structural SIMilarity (SSIM) index8 and root-mean-square error (RMSE) were calculated for each motion scan (after a rigid registration to the no motion reference volume), where the “no motion” scan was used as a reference. Reconstruction time with motion correction was 47 seconds per coil which can be parallelized.Results
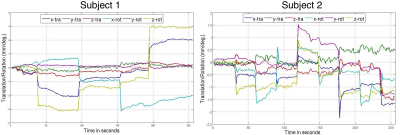
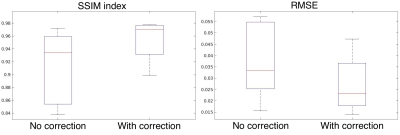
Figure.1 shows sample motion traces recorded from two different subjects. Figure.2 shows sagittal and axial slices from subject-1 from the “no motion” scan, “motion” scan and “motion” scan with retrospective correction applied. At the bottom of the figure the difference image between the no motion reference scan is shown. Figure.3 shows similar results for subject-2. Figure.4 shows the zoomed images from subject-1 (top) and subject-2 (bottom) highlighting the effect of motion correction on the fine details of the cortex. When all twelve scans were analyzed, SSIM increased from 0.91±0.05 (without correction) to 0.96±0.03 with retrospective motion correction using data from the EM sensors. RMSE reduced from 3.78±1.51 to 2.74±0.11 when motion correction was applied.Discussion
The retrospective correction using EM sensor data improved the image quality both qualitatively and quantitatively in all 12 motion corrupted scans. The data included motions up to 7mm of translation and 5o of rotation. Since no reacquisition was implemented, in the cases where motion free time is low (i.e subject-2), remaining ghosting artifacts can be seen in the motion corrected images (see Figure.4).Conclusion
The presented results show that motion traces from the EM sensors can be used to retrospectively improve image quality. The EM tracking system can be used with any imaging sequence currently being employed in clinical studies. One challenge that remains is the sensor placement on the head and the effects of skin motion that needs to be considered to further improve image quality. For this work we assumed that GRAPPA coefficients can be reliably estimated since the motions are small but the effect of motion on coil sensitivities will be investigated in future work.Acknowledgements
This research was supported in part by the grants NIH-5R01EB019483, NIH-4R01NS079788 and NIH-R44MH086984.References
1. Afacan, O., Erem, B., Roby, D.P., Roth, N., Roth, A., Prabhu, S.P. and Warfield, S.K., 2016. Evaluation of motion and its effect on brain magnetic resonance image quality in children. Pediatric radiology, 46(12), pp.1728-1735.
2. Zaitsev, M., Dold, C., Sakas, G., Hennig, J. and Speck, O., 2006. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. Neuroimage, 31(3), pp.1038-1050.
3. Pipe, J.G., 1999. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magnetic resonance in medicine, 42(5), pp.963-969.
4. Tisdall, M.D., Hess, A.T., Reuter, M., Meintjes, E.M., Fischl, B. and van der Kouwe, A.J., 2012. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magnetic resonance in medicine, 68(2), pp.389-399.
5. Maclaren, J., Herbst, M., Speck, O. and Zaitsev, M., 2013. Prospective motion correction in brain imaging: a review. Magnetic resonance in medicine, 69(3), pp.621-636.
6.Fessler, J.A. and Sutton, B.P., 2003. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Transactions on Signal Processing, 51(2), pp.560-574.
7. Gallichan, D., Marques, J.P. and Gruetter, R., 2016. Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T. Magnetic resonance in medicine, 75(3), pp.1030-1039.
8. Wang, Z., Bovik, A.C., Sheikh, H.R. and Simoncelli, E.P., 2004. Image quality assessment: from error visibility to structural similarity. IEEE transactions on image processing, 13(4), pp.600-612.
Figures