2651
Optical prospective motion correction for brain imaging at 7T without a mouthpiece1Department of Radiology, Stanford University, Stanford, CA, United States, 2MR Applied Science Lab, GE Healthcare, Menlo Park, CA, United States
Synopsis
The advancements in signal to noise ratio, contrast, and resolution enabled by high-field MR systems provide great potential for visualizing more nuanced brain anatomy. However, in order to translate these advancements to the discovery and clinical implementation of novel neuroimaging biomarkers, motion artifact resulting from long scan times must be addressed. Here, we demonstrate proof-of-concept of a novel prospective optical motion tracking and correction system using a coil-mounted camera without a mouthpiece, visualizing an optical marker placed on the cheek of human subjects in a 7T MR system.
Introduction
High-field MRI systems, such as 7 tesla (7T) scanners, are often used to produce images with higher SNR and spatial resolution than conventional field-strength MR systems. However, achieving both high SNR and high resolution requires long scan times, often resulting in image artifacts due to subject motion. Addressing this motion artifact at 7T is a pivotal step in using these systems to identify novel biomarkers of disease.
Prospective motion correction methods, which compensate for motion in real-time, often rely on optical monitoring using a camera to obtain the required tracking data. Several studies1,2 have successfully used MR-compatible cameras mounted inside the bore of the magnet for optical tracking at 7T. However, these studies use mouthpieces to mount the tracking marker and large bore-mounted cameras in order to achieve the required line-of-sight for successful head tracking. Other work3 has demonstrated feasibility of a coil-mounted camera system for optical tracking motion correction at 1.5T and 3T, which is more practical than using a mouthpiece for patient studies. The goal of the present study is to translate this latter approach to a 7T scanner, enabling prospective tracking at 7T in patients, facilitating discovery of novel neuroimaging biomarkers.
Methods
A single MR-compatible camera was mounted onto a Nova Medical 2 Tx/32 Rx head coil for a 7T GE MR950 scanner (Figure 1). The location of the camera was calibrated using the method described in [3]. Initial validation of the calibration and techniques were conducted using papaya phantoms, with deliberate rotational motion applied using a wooden rod. The same sequence was acquired twice, once without and once with motion correction. The ability of the system to correct for deliberate, rotational-drift motion in human subjects was tested in this study by placing the marker between the subject’s nose and cheek (Figure 1B), since direct line-of-sight to the forehead was not possible due to the location of the Tx coil. One subject was asked to produce continual rotational-drift motion, rotating ~5-6 times per minute, during a 2D GRE sequence (2D FAST, TR = 515, TE = 20, Resolution = 0.146mm x 0.146mm x 1.5mm, 11 slices, scan time 08:36), acquired once uncorrected and once corrected. To validate the system with involuntary motion, two compliant subjects were scanned twice with the same 2D GRE sequence.
Results
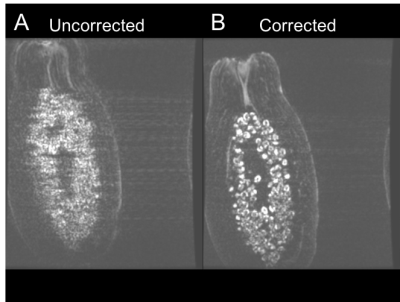
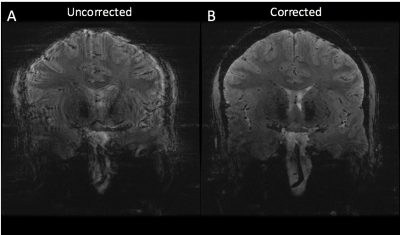
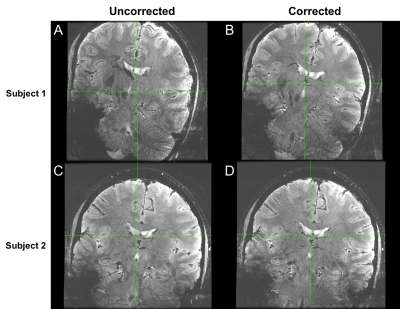
Figure 2 shows the results of the initial phantom validation study, demonstrating clear improvement of motion artifact in the corrected acquisition. Figure 3 demonstrates the results of the deliberate motion study conducted in a human subject. Here, again, significant improvement can be seen in the motion-corrected acquisition. Figure 4 subsequently displays the results of each of the two involuntary motion studies in human subjects. The results from the first study demonstrate clear improvement in the corrected acquisition. The results from the second study do not demonstrate a noticeable visual difference between the two acquisitions. Notably, the motion correction did not introduce marked motion artifacts in any of the corrected acquisitions.Discussion
The results of this study demonstrate the feasibility of using a prospective optical motion tracking system at 7T using a coil-mounted camera and marker attached to the subject’s face. The ability to correct both deliberate and involuntary motion during scans has been demonstrated in human subjects. In one subject, no significant benefit was seen in the motion-corrected acquisition, likely due to the non-optimal set-up of this preliminary system. The placement of the marker forces the subject's head to be rotated and tilted downward within the coil for line-of-sight between the camera and the marker, resulting in the oblique prescription seen in Figures 3-4. Additionally, the marker's placement on the cheek may result in sensitivity to facial twitches, which do not reflect global head movements and may be falsely corrected. However, the promising results demonstrated here provide strong evidence for the usability of such a system and are motivation for further improvements, including adapting the camera design to be mounted between Tx and Rx shells, allowing for the marker to be placed on the subject’s forehead. This is expected to provide a more rigid coupling between the subject’s head and the marker, improving system performance.Conclusion
These results demonstrate the potential of this system to address the presently unresolved issue of motion artifact in neuroimaging at 7T. By implementing and optimizing this system in both research and clinical settings, there is strong potential for resolving novel neuroimaging biomarkers of aging and disease. Our initial application will focus on high resolution imaging of the hippocampus in patients with Alzheimer’s disease to uncover novel mechanisms of neurodegeneration.Acknowledgements
The authors would like to acknowledge research support by GE Healthcare and by NIH P41 EB015891, NIH S10 RR026351-01A1, and NIH R01 EB 011654.
References
1. Maclaren, J., et al. "Measurement and correction of microscopic head motion during magnetic resonance imaging of the brain." PloS one 7.11 (2012): e48088.
2. Stucht, D., et al. "Highest resolution in vivo human brain MRI using prospective motion correction." PloS one 10.7 (2015): e0133921.
3. Maclaren, J., et al. "Prospective motion correction using coil‐mounted cameras: Cross‐calibration considerations." Magnetic Resonance in Medicine (2017).
Figures