2648
High resolution imaging at 7T using interleaved prospective motion correction (iMOCO)1Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark, 2Philips Healthcare, Copenhagen, Denmark, 3Center for Magnetic Resonance, Department of Electrical Engineering, Technical University of Denmark, Lyngby, Denmark
Synopsis
Subject motion is a major problem in MRI, leading to less diagnostic information in the clinic and lowering data quality in research. Especially at high field, the relatively long scan times applied for high resolution imaging makes motion one of the major challenges. A promising solution is to update the field-of-view in real time based on tracking with MRI-based navigators. Here we show an implementation for prospective motion correction using MRI navigators at 7T. The framework was very flexible, as the navigator and target sequence are simply defined as two different scans, which can be interleaved at any sequence level.
Introduction
Previous motion correction studies at 7T have used optical tracking for real time correction [1], or navigators for retrospective corrections [2]. Optical tracking is, however, especially challenging to make patient compliant at 7T due to the local transmit head coils obstructing the line of sight. Retrospective corrections have severe limitations, especially with through-plane motion in multi-slice sequences. We here demonstrate real-time motion correction at 7T with MR navigators, using a recently developed interleaved scanning framework for interleaved real-time motion correction (iMOCO).Methods
The iMOCO framework allows instantaneous switching between sequences at any time point in the execution [3,4]. Therefore, the navigator sequence and host sequence were simply defined as two subsequent sequences which are started simultaneously.
In the navigator sequence, the prospective motion correction (PMC) functionality is used. The PMC functionality registers the latest acquired dynamic volume to the first, and uses the realignment parameters for updating the translation offsets and orientation matrix of the host sequence in real-time.
Both water-based (vNAV[1] 7x7x7mm, EPI=31, halfscan=0.87x0.87) and fat-selective (fat-NAV[2], 7x7x7mm, EPI=3, SENSE=2x2, halfscan=0.89x0.85) volumetric navigator sequences were investigated in two high resolution host sequences; 3D MP-FLAIR and multi-slice T2 weighted turbo spin echo. Especially the multi slice T2 weighted turbo spin echo (MS-T2 TSE) has shown to be highly sensitive to motion artifacts in clinical studies. 3D MP-FLAIR was acquired with an echo train length of 200, TE=389, TR=5000, 0.7x0.7x1.4mm resolution in 8:20 min. The MS-T2W TSE had an echo train length of 9, TE=60, TR=3373, FOV=220x190, resolution = 0.5x0.5x1, 32 slices, total scan time 8:22 min. Reacquisition was performed if a motion update exceeded 1 mm of equivalent displacement.
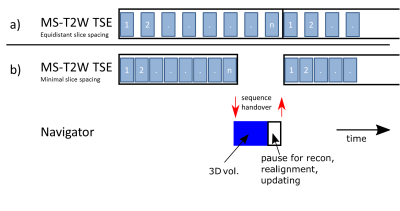
For the 3D sequences, the navigator was inserted in the long relaxation after a shot. The MS-T2 TSE sequence inherently has some time gaps due to SAR limitations. Although a segmented navigator sequence could fit into the native gaps, here we choose minimal slice spacing to generate a large gap in the end of each TR a non-segmented navigator sequence could fit into (see Figure 1).
All experiments were performed on a 7T MRI system (Achieva, Philips, Best, Netherlands) with a dual transmit coil and a 32-channel receive head coil (Nova Medical, Wilmington, MA, USA). All experiments were performed in accordance to the local ethical guidelines.
Results
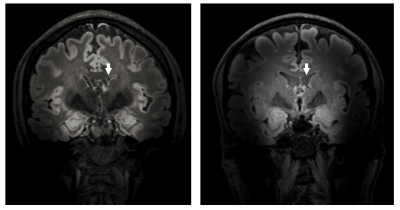
Figure 2 shows the results of using a vNAV (left) and a fat-NAV (right) in the 3D FLAIR sequence. Although the navigator is performed at the end of the relaxation curve, and a 1 degree nominal flip angle is used, a distortion of the steady state magnetization is observed. With fat-selective navigators, this problem is circumvented.
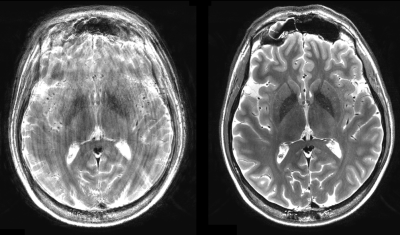
Figure 3 shows MS-T2-TSE sequences (which are highly sensitive to motion) with and without motion correction. The iMOCO restores the image quality despite subject motion.
Discussion
The iMOCO framework makes it flexible to add prospective motion correction with volumetric navigators to almost any sequence. The flexibility is demonstrated here by the incorporation of both vNAVs and fat-NAVs into high resolution T2 weighted multi-slice and 3D sequences at 7T. In these sequences, fat-navigators seem to be superior to non-selective navigators due to the increased T1 times and B1 field inhomogeneities at 7T.Acknowledgements
This research is supported by the Danish Council for Independent Research [grant no. 6111-00349A].References
[1] D. Stucht, K. A. Danishad, P. Schulze, F. Godenschweger, M. Zaitsev, and O. Speck, “Highest Resolution In Vivo Human Brain MRI Using Prospective Motion Correction,” PLoS One, vol. 10, no. 7, p. e0133921, 2015.
[2] D. Gallichan, J. P. Marques, and R. Gruetter, “Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T,” Magn. Reson. Med., vol. 75, no. 3, pp. 1030–1039, 2016.
[3] M. Andersen, V. Boer, A. Marsman, and E. Petersen, “A generalized prospective motion correction framework for improved spectroscopy, structural and angiographic imaging,” Proc. Intl. Soc. Mag. Reson. Med., p. 3934, 2017.
[4] M. Henningsson, G. Mens, P. Koken, J. Smink, and R. M. Botnar, “A new framework for interleaved scanning in cardiovascular MR: Application to image-based respiratory motion correction in coronary MR angiography,” Magn. Reson. Med., vol. 73, no. 2, pp. 692–696, Feb. 2015.
[5] M. D. Tisdall, A. T. Hess, M. Reuter, E. M. Meintjes, B. Fischl, and A. J. W. Van Der Kouwe, “Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI,” Magn. Reson. Med., vol. 68, no. 2, pp. 389–399, 2012.
Figures